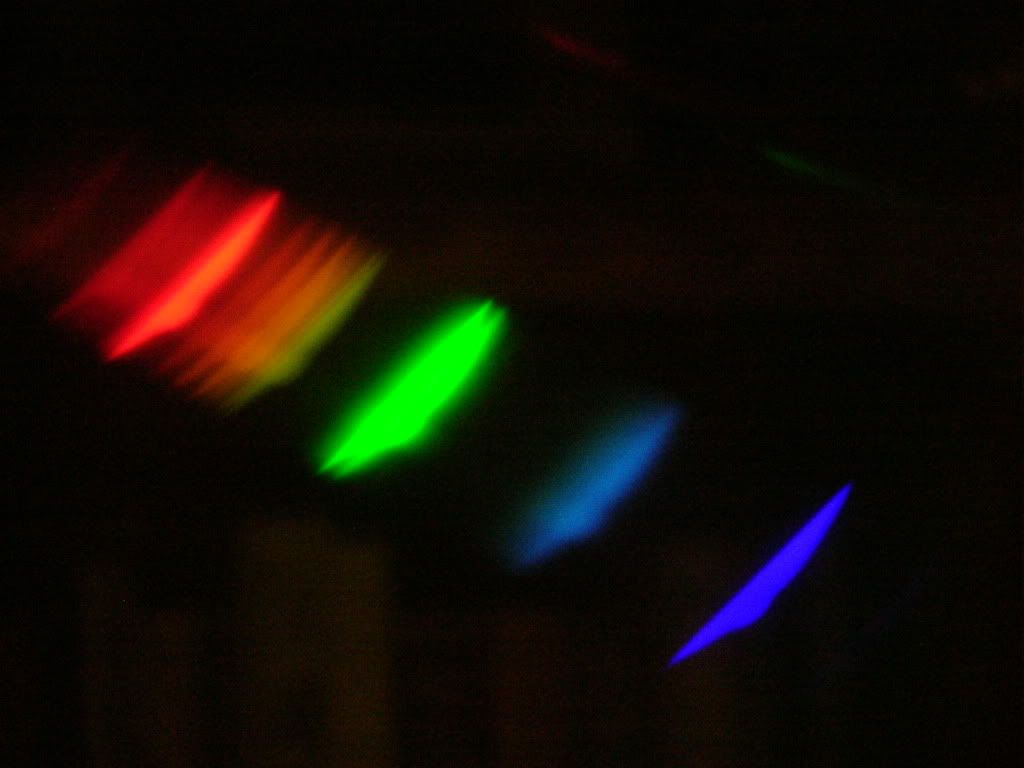
Posted on 03/30/2011 7:35:54 PM PDT by neverdem
Light bulbs rely not only on simple materials but on esoteric ions and compounds. And while we take their emissions, visible light, for granted, the inner workings of these deceivingly simple gadgets depend on the complex behavior of electrons.
We’ll discuss four types of light bulbs:incandescent bulbs, halogens, fluorescent lights (including CFL’s) and LED’s.
A) INCANDESCENT BULBS
The light bulb of the short-lived variety, is the traditional tungsten incandescent bulb. Inside the glass, electricity flows through a thin filament of the element tungsten (chemical symbol, W, for its old name wolfram).
Because the wire is so thin, resistance is high, and it raises the temperature of the tungsten wire, so chosen because of its high melting point of 3410 oC. At the bulb’s temperature, which is about 1000o cooler, excited electrons that return to lower energy states release photons of a frequency that is visible to the human eye. The radiation is intense in the red to yellow regions but compared to daylight, the
spectrum of an incandescent light bulb is very weak in the 400 to 500 nm region (blue). This would be nice to verify with a prism, and is the reason that plants don’t do as well if grown under such light. Although the heat is not sufficient to melt the tungsten it would certainly fry the heck out of it in an oxidizing atmosphere. Thus manufacturers replace oxygen with a mixture of the less reactive nitrogen and the noble gas argon. Note that a vacuum would not be a good solution because the tungsten would vaporize even more easily and dramatically shorten the bulb’s lifespan.
Even within an argon-nitrogen atmosphere, however, the heat causes some of the tungsten to sublimate. Some of it returns to the wire as it bounces off the argon gas, but a good deal ends up on the glass. This is one of the reasons incandescent bulbs tend to get darker with increased use. The glass suffers more abuse from plain old electrons which fly off the filament as if it were a cathode ray tube from a conventional television set. Such electrons cause tiny black spots to appear. These first caught Edison’s attention, but he had never time for further investigations; otherwise, as David Bodanis suggests, Edison may have discovered electrons before J.J. Thomson. To create a more diffuse light but perhaps in an attempt to camouflage all the future damage, manufacturers treat light bulb glass with hydrofluoric acid one of the few acids that can attack glass) which creates that familiar frosty look.
B)HALOGENS
Eventually the tungsten wire becomes so thin, that it snaps, breaking the circuit and sending you off to the hardware store. At one point someone got tired of the bulb’s short lifespan and invented the halogen light bulb. This still uses tungsten but along with argon it includes a small amount a halogen gas, namely chlorine. The reactive gas combines with the tungsten vapour and deposits it again on the filament. In other words it recycles the tungsten, rather than letting it wastefully deposit on the glass. Of course, it is very unlikely that the metal will be perfectly and evenly replaced all along the coiled filament. Weak spots eventually develop, and the coil still breaks, but it takes a lot longer, and halogen bulbs outlive their incandescent counterparts. The glass has to be able to withstand higher temperatures, so they use a purer form of silicon dioxide, one that unfortunately gets ruined by oils on our skin. If these bulbs are mishandled as such, the grease should be washed away with alcohol.
C)FLUORESCENT BULBS
To avoid wasting energy in the form of heat, fluorescent lights, ubiquitous in schools and other institutions, operate by a totally different principle. They contain a small amount of mercury(Hg), which emits ultraviolet light when excited by electrical energy. The story cannot end there because ultraviolet(UV) is invisible to the human eye. The walls of the bulbs are coated with a phosphor, usually a halophosphate such as Ca5(PO4)3(F,Cl) with ions of Sb3+ and Mn2+ that absorb the UV radiation. The excited electrons then release visible light, compliments of fluorescence. In this form of luminescence, an excited electron returns from a specific molecular orbital to a lower one without inverting its spin. The resulting light has the bulk of its intense wavelengths in the yellow and blue regions. But relative to natural light, fluorescence is weak in the red regions. Plants will again remain "unhappy", unless you buy more expensive fluorescent lights which try to compensate for this weakness by substituting antimony and manganese ions in the phosphor with europium and terbium ions.
Next we come to the smaller version of type 3 bulbs, compact fluorescent bulbs(CFL's), which are overrated for three reasons:
(1) Practically, the CFL’s are not as bright as halogens and although they match the light intensity of incandescent bulbs, they take a while to reach their peak intensity.
(2) They were not designed for cold climates, where the traditional bulb’s inefficiency is less of a drawback. The heat generation is actually desirable for about 9 months of the year in the northern states and Canada because it means the main heat source in the home does not have to work as hard.
(3) It’s ironic that something marketed as an environmental savior actually contains mercury. According to Environment Canada, the Hg content varies from 1 to 25 mg per bulb. There are about 115 million American households. If each household breaks 3 bulbs per year either by accident or indirectly by sending them to a landfill, then between 300 kg and 9000 kg of mercury (one significant figure) are added to the environment in the United States alone. The annual mercury emissions from all sources in the United States are estimated at 43 700 kg ( over 12000 kg from Alaska).
D)LEDS
To gain insight into how an LED (light emitting diode) bulb works we need to be reminded that a diode consists of two adjacent wafers of silicon doped with different impurities. The latter do not have the same valence number as silicon. If the impurity or "doping agent" is short of an electron(for example, boron), its wafer will receive an electron from the wafer with the opposite problem(example arsenic). Since electrons are stepping down from a higher energy level, photons are released. The energy gap is usually small and will only emit in the infrared, but it’s still useful if you want to use the remote control to turn off your daughter’s music channel.
To get visible light you need to get away from the classic boron-arsenic combo representing a valence of 3 and 5, respectively. If aluminum and gallium(each with a valence of 3) replace boron, one can create a red LED. If indium replaces aluminum, the transition energy increases, and blue light is released. The third primary color is created by replacing arsenic with another valence 5 element, phosphorus, and combining it with aluminum and gallium. A white color can result from combining all three recipes or by coating the bulb with a phosphor.
Although there are still technical challenges ahead, LED lights will probably replace CFC's. But perhaps not to add too much arsenic to the environment, we should also use incandescent light or simply wait for sunrise to read science.
References
University Corporation for Atmospheric Research http://www.ucar.edu/news/releases/2007/nicc-table.shtml
US Census http://www.census.gov/prod/1/pop/p25-1129.pdf
Environment Canada http://www.ec.gc.ca/mercure-mercury/default.asp?lang=En&n=2486B388-1
Efficiency of CFL’s http://www.cbc.ca/news/canada/manitoba/story/2009/03/04/mb-light-bulbs.html
US Department of Energy http://www1.eere.energy.gov/buildings/ssl/how.html
http://science.howstuffworks.com/light-bulb2.htm
Bodanis, David. Electric Universe. Crown. 2005
Britannica. DVD edition. 2000
Haber Schaim and Al. PSSC Physics. Heath. 1971
All I know are these new light bulbs suck. I have difficulty reading under them.
And we have far too much sex on the television. I keep falling off.
Seriously, if the light bulbs suck, you probably have them screwed in reverse. Screw them in the opposite direction, and they will emit, instead of sucking. No wonder you have difficulty reading.
Then you're not buying the ones with the appropriate spectral output. The article is pretty simplistic in its description of CFL's. There are several varieties with different "color temperatures". The normal CFL's have more blue emission, but there are "warm white" versions that duplicate incandescent spectra more closely.
If you have your light-bulb installed to emit, you have a Light Emitting Diode, or LED. If you have your light-bulb installed to suck, you probably have LSD.
What if, say, each of 100,000 households breaks about a dozen CFLs all within a minutes of each other by sending them to a wasteland, such as northeast coastal Japan?
This is an environmental disaster in the making,
Remember asbestos? They put it in everything when I was a child. I remember being told in school have safe it was because it was permeated with asbestos - the floors, the ceilings.
Just watch. In 20 or so years all of a sudden it’s going to become a huge environmental emergency (all that Hg) and the scam artists that live off the government will suddenly start up all these “Toxic metal removal” companies. And get money from the public purse to do it.
Al Gore’s next scam.
They crap out quickly in my part of the world (high and cold), and the light from them is dim through their short lives. LEDs are better for here but mucho expensive.
Each lab station in my high school science classroom had a plastic bottle full of mercury. I don't remember what science projects the mercury was for, but we enjoyed rolling it around in our palms. Somehow we've all reached our late 40s apparently unscathed.
My nephew is working on LEDs at Phillips Luminesce after 20+ years with HP and it’s spin offs developing things like hand held barcode scanners to the camera modules for cell phones. Phillips has experimental LED street lighting in a couple of towns in France that I know of...
Pure mercury is actually pretty safe because the body doesn’t absorb it well (but it’s nasty if ingested). The real danger comes from mercury compounds that can be absorbed through the skin or lungs.
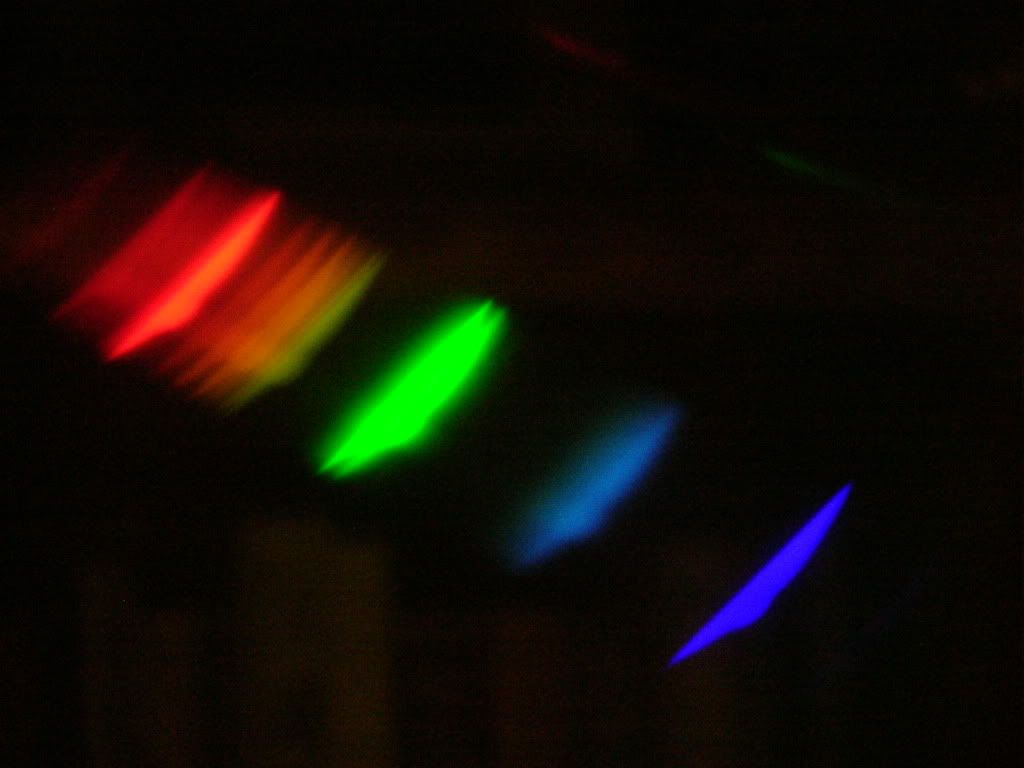
Edison could be said to be the grandfather of electronics. During his investigation of aging effects in his incandescent lamps, he added a small metal plate adjacent to the filament (but not touching it), and brought a connection to it out the side of the bulb.
[Edison was the ideal guy to do this, because in the 1880's nobody was better at building evacuated glass bulbs with things like filaments and plates and bringing their connections out through gas-tight seals in the glass.]
Anyway, he noticed that you could get an electrical current to flow from the plate to the filament through the vacuum, provided you connected the + end of a battery to the plate and the - end to one of the filament leads. I don't think that anybody else had thought, at that stage, that such a thing could happen.
Oh, and one other thing about this phenomenon (that came to be called the "Edison Effect"): current would not flow in the other direction. You could hook up the battery - to the plate and its + to the filament, and nothing would happen.
Bear in mind that Edison's electrical system was exclusively DC--direct current. He had no use for a device that admitted current flow in one direction but not the other, because any element in his system would always be passing current in one direction only.
So, after patenting a device that made trivial use of his gadget, he set it aside. A few physicists here and there got copies of the gadget and investigated it, and then they too set it aside.
Meanwhile, radio was invented by the likes of Tesla, Marconi, and Preece.
One of the early problems with radio transmission was that the first receivers were incredibly insensitive. An example was the "coherer" of Edouard Branley, an insulating tube filled with fine iron filings, which could be made to conduct when furnished with a pulse of radio frequency energy from the aerial. As a result of the deafness of the available receivers, transmitters in the first decade of the 20th centry were being built to gargantuan proportions in order to get enough power into the distant receivers for them to operate. In effect, the receivers were directly transmitter-powered.
So the search was on for a better detector, the heart of the receiver that could turn radio waves from the aerial into currents that could operate things such as telegraph sounders.
Around 1904, John Ambrose Fleming, an English physicist, pulled a dusty Edison apparatus out of a drawer. He reasoned that a device that could pass current in only one direction--that is, it could rectify--might the the ticket to a better detector. In a short time, he hooked it up in a receiver and got an astounding improvement in sensitivity.
Although this was not the only innovation in early radio receivers (the Galena crystal and cat's whisker were another), it set radio technology on a rapid growth curve.
About four years later, a young Stanford PhD on the west coast by the name of Lee DeForest reprised Edison's experiments of two decades previous, in order to better understand the behaviour of what was now being called the "Fleming Valve." This time, he inserted a third element between filament and plate which he called a "grid." He discovered that varying potentials on the grid controlled the flow of current from the plate to the filament, and that he could control a given amount of current with a small amount of voltage. Thus, his gizmo was the first electronic device to exhibit gain. And thus not just radio, but electronics itself was finally born.
Had Edison done his work just ten years later, and had he been developing AC systems, he might have been the father and not just the grandfather of electronics. As it was, DeForest was the father and Fleming, you might say, was the midwife.
Postscript: In 1897, amidst the early development of radio, English physicist J. J. Thompson used an early version of the cathode ray tube to discern the nature of the effect that gave the tube its name. In doing so, came up with convincing evidence that the current through the vacuum was being carried by tiny, tiny particles which he called "electrons." Further experiment revealed the charge and mass of the electron, and established that it was a constituent of the atom--the first subatomic particle to be discovered. It was also shortly established that the electron was also the carrier of electric current through ordinary conductors as well.
One final discovery. According a convention in electrical research that was long established even in 1897, Thompson found that the charge on the electron was negative. Therefore, in the various electron devices here described, the "conventional current" was flowing in the device from plate to filament, but in actuality the negative electrons were flowing from filament to plate. A fact that confuses beginning EE students to this very day. ≤}B^)
RE your post 17: Fascinating, thanks for the lesson!
Thanks for the history.
I wonder if the young Tea Party hotshots in Congress are going to bring this up and relieve us of the curly light bulbs. I don't hear a peep from them about this.
I know they're handling some other things of importance like the budget and war and all that stuff....but how long would it take to slip in a little light bulb legislation?
I would even write it for them.
Leni
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.