
Posted on 06/24/2013 12:56:35 AM PDT by neverdem

The effects of relativity can be seen in everyday phenomena © Shutterstock
Why is mercury a liquid at room temperature? If you ask that question in a school classroom you will probably be told that relativity affects the orbitals of heavy metals, contracting them and changing how they bond. However, the first evidence that this explanation is correct has only just been published.
An international team led by Peter Schwerdtfeger of Massey University Auckland in New Zealand used quantum mechanics to make calculations of the heat capacity of the metal either including or excluding relativistic effects. They showed that if they ignored relativity when making their calculations, the predicted melting point of mercury was 82°C. But if they included relativistic effects their answer closely matched the experimental value of -39°C.
Relativity states that objects get heavier the faster they move. In atoms, the velocity of the innermost electrons is related to the nuclear charge. The larger the nucleus gets the greater the electrostatic attraction and the faster the electrons have to move to avoid falling into it. So, as you go down the periodic table these 1s electrons get faster and faster, and therefore heavier, causing the radius of the atom to shrink. This stabilises some orbitals, which also have a relativistic nature of their own, while destabilising others. This interplay means that for heavy elements like mercury and gold, the outer electrons are stabilised. In mercury’s case, instead of forming bonds between neighbouring mercury atoms, the electrons stay associated with their own nuclei, and weaker interatomic forces such as van der Waals bonds hold the atoms together.
In the 1960s, Pekka Pyykkö, now at University of Helsinki, Finland, discovered that gold’s colour was the result of relativistic effects. He showed that the lower energy levels of the 6s orbital of gold means that the energy required to excite an electron from the 5d band lies in the visible rather than UV range of light. This means that gold absorbs blue light, while reflecting yellow and red light, and it is this that gives the metal its characteristic hue. If the energies of the two bands were calculated without including relativistic effects, the energy required is much greater. Further calculations have subsequently shown the influence of relativity on the colour and bond lengths of heavy metal compounds, as well as its importance in catalysis. However, the low melting point of mercury could still only be described as ‘probably’ due to relativistic effects.
‘At a hand-waving, speculative level, this idea [that relativity affects the melting point of mercury] has been around since the late 1970’s,’ explains Pyykkö, who was not involved in the work, ‘but this is the first quantitative proof.’
Schwerdtfeger’s team, in particular, have been working on the problem for couple of decades. The reason for the delay, he explains, was that until recently computers could not complete the powerful calculations the team performed. ‘A lot of computer time was required,’ he adds, ‘and the algorithms used are more efficient nowadays.’
But beyond making it into the textbooks, which this work will definitely do, Schwerdtfeger hopes that by showing that his approach works it can be used to calculate the melting points of other metallic systems.
But more importantly, next time a teacher gets asked about one of the most conspicuous examples of relativity, they’ll know there’s evidence to back up their explanation.
News to me. Thanks for posting.
Interesting article. Thanks for digging it up. I wonder what is the linkage between mercury’s purported toxicity and its unique physical properties?
TC
I did not post the article, neverdem did. I don't know the answer to your question, but I do know that the toxicity of mercury compounds has been proved. Perhaps you were referring to elemental mercury.
Fixed it.
I’ve read that elemental mercury is not particularly toxic. Some mercury compounds, that can be absorbed during digestion, destroy the liver. The ones that cannot be digested are harmless. Mercury compounds used to be used as a medication to alleviate the symptoms of syphilis. Don’t try this at home.
I used to love playing with this stuff back in the ‘60’s. I don’t think it ever harme...ooo a piece of candy.
My college chemistry prof said that all metals are poisonous.
LOL I use to play with it also, usually when a thermometer got dropped on the floor and tiny beads of mercury were all over the floor....mom would say, go clean up the mercury, but I also was fascinated by how it reacted and became little dots and when pushed together, clung together....When I told my son and grandson, they laughed themselves silly, thinking just touching mercury was deadly...but dad did warn me to get out of the room if a florescent bulb broke....it has Mercury vapors....thats not good....
That's right - they helped reduce the chancres or sores.
Of course they were toxic in their own right.
Mercury was considered a state of the art cure for health problems in the early nineteenth century. The Lewis and Clark expedition took along a large supply.
This fact allowed modern archaeologists to precisely map out the expedition's route. They just went to potential sites and tested the soil. Whenever they found mercury contamination they knew they had located a latrine.
It is sometimes suspected that Lewis killed himself in the throes of syphilitic dementia. Or perhaps it was dementia caused by mercury poisoning. Or dementia caused by malaria. Or dementia caused by opium. Or it's possible that he was murdered.
Stories like Lewis's make me feel better about modern medicine. Although of course modern medicine has its own horror stories.
Water can a kill a person if they drink enough of it.
OTOH, we couldn't live without iron, or quite a few other metals.
There is a book, “Heavenly Intrigue” that argues that Tycho likely died of mercury poisoning administered by Kepler. Tycho, besides running an observatory in Prague, had a medical shop, where he formulated mercury compounds to treat a disease he contracted while a student, around the same time he lost his nose in a sword fight. (He was a nobleman student and had a chaperon, who was probably as drunk and whore-infested as he was.)
The formulary produced various mercury compounds in the process, some of which were known to be toxic. Kepler certainly had means, motive and opportunity. No one benefited from Tycho’s very opportune death as much as Kepler, for whom it was a godsend, both professionally and financially.
"How does he smell?"
"Awful!"
They mixed mercury with iodine and called it merthiolate. I had my hand soaked in in every day over a 2 week period to treat a splinter that went all the way through my palm. The company doctor didn’t stitch it up after removing the splinter and squeezing out the “white stuff” until he was finished with the mercury treatment. Remember company doctors? Obamacare will bring them back, I’m sure. Med students who had “C” average in school.
“Dr. Johnson, you smell awful!”
“No, Madam, I stink, you smell.”
Apparently, some idiot just wanted to make a splash about Tycho Brahe.
From Wikipedia:
The scientists, led by Dr Jens Vellev, analyzed Tycho’s beard hair once again. The team reported in November 2012 that not only was there not enough mercury present to substantiate murder, but that there were no lethal levels of any poisons present. The team’s conclusions was that “it is impossible that Tycho Brahe could have been murdered.”
Unless you physically work with inorganic mercury or its various salts, I don't think you have much to worry about. Playing with elemental mercury was a pretty big fad in the 1960s.
IMHO, mercury's low melting point has nothing to do with the covalent bonds it makes with methyl, ethyl or other organic groups in, e.g. dimethyl mercury, diethyl mercury, etc. Humans need to worry about consuming too much fish and shellfish or they can accumulate too much dimethyl mercury or diethyl mercury. I don't think it matters if they come from fresh or salt water.
Most of mercury gets in the atmosphere from burning coal. Its one of the main medical reasons that they want to kill coal. Inorganic mercury comes down wherever it rains. Various organisms convert to the toxic diethyl and dimethyl forms, and it gets more concentrated as it goes up the food chain.
The following links a fairly recent review article:
Mercury Toxicity and Treatment: A Review of the Literature
Abstract
Mercury is a toxic heavy metal which is widely dispersed in nature. Most human exposure results from fish consumption or dental amalgam. Mercury occurs in several chemical forms, with complex pharmacokinetics. Mercury is capable of inducing a wide range of clinical presentations. Diagnosis of mercury toxicity can be challenging but can be obtained with reasonable reliability. Effective therapies for clinical toxicity have been described.
>> Most of mercury gets in the atmosphere from burning coal. Its one of the main medical reasons that they want to kill coal. <<
Your science is accurate, but your politics are not. Present policies are already greatly reducing mercury emissions, and they can be reduced far further, largely ending the problem, without having to “kill coal.”
Coal is domestic, and hence inevitably commoditized; there’s no profit. The big oil industries and the OPEC nations want to kill coal because our reliance on foreign oil from dictatorships makes it impossible to commoditize.
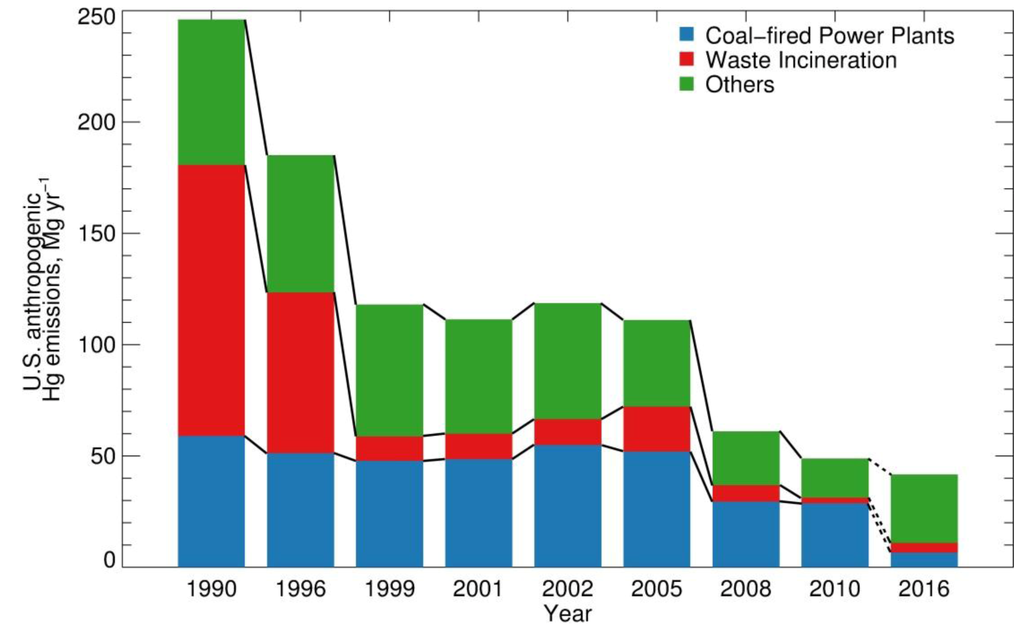
Notice the decline from fuel emissions from 170 mg/Yr to under 20.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.