Posted on 05/29/2020 9:44:40 PM PDT by SeekAndFind
The use of Real World Evidence in Covid-19 is growing, and that’s a good thing. Properly implemented, RWE has the potential to deliver solid results faster than Randomized Controlled Trials, as I explained in a .css-mckguv{-webkit-transition:background 0.25s var(--ease-in-out-quad),color 0.25s var(--ease-in-out-quad);transition:background 0.25s var(--ease-in-out-quad),color 0.25s var(--ease-in-out-quad);color:var(--theme-ui-colors-accent,#6166DC);}.css-mckguv:visited{color:var(--theme-ui-colors-accent,#6166DC);opacity:0.85;}.css-mckguv:hover,.css-mckguv:focus{-webkit-text-decoration:underline;text-decoration:underline;}previous blog post.
So I couldn’t miss the largest observational study published to date on the effects of (hydroxy-)chloroquine, in 96 032 hospitalised Covid-19 patients, from an international registry comprising 671 hospitals in six continents:
Mehra, M. R., Desai, S. S., Ruschitzka, F., & Patel, A. N. (2020). ‘Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis’. The Lancet.
Unfortunately, this paper is an example of Real World Evidence and Big Data implemented in the wrong way. Data is powerful, but not a magic bullet, and this kind of low-quality literature is my inspiration for launching the COVIND Consortium of Individual Patient Data.

In this Lancet piece, I identified 3 flaws:.css-1rpojxi{list-style:none;counter-reset:list;color:var(--theme-ui-colors-articleText,#08080B);position:relative;padding:15px 0 30px 30px;-webkit-transition:background 0.25s var(--ease-in-out-quad),color 0.25s var(--ease-in-out-quad);transition:background 0.25s var(--ease-in-out-quad),color 0.25s var(--ease-in-out-quad);margin:0 auto;font-size:18px;width:100%;max-width:680px;}@media (max-width:66.875em){.css-1rpojxi{max-width:507px;}}@media (max-width:45.9375em){.css-1rpojxi{max-width:486px;padding-left:0px;}}@media (max-width:33.75em){.css-1rpojxi{padding-left:20px;}}.css-1rpojxi li{position:relative;padding-bottom:15px;}@media (max-width:45.9375em){.css-1rpojxi li{padding-left:30px;}}@media (max-width:33.75em){.css-1rpojxi li{padding-left:30px;}}@media (max-width:45.9375em){.css-1rpojxi li p{padding:0;}}.css-1rpojxi li >:not(ol,ul){display:inline;}.css-1rpojxi li::before{width:3rem;display:inline-block;position:absolute;color:var(--theme-ui-colors-articleText,#08080B);}.css-1rpojxi li::before{content:'';position:absolute;left:-30px;top:8px;height:8px;width:8px;background:var(--theme-ui-colors-articleText,#08080B);}@media (max-width:45.9375em){.css-1rpojxi li::before{left:0;}}
First, this study is not reproducible. Authors did not release their data, and it’s not even in their plans:

They argue legal pretexts, but it’s still a bad professional practice. Their data is a black box. In terms of reproducibility, this paper doesn’t meet community standards we have in machine learning, where most authors release their data and code on GitHub, and sometimes even build a ready-made container for one-click reproducibility on Google AI Hub, or other cloud platforms.
As expected, this data secrecy implies some weird stuff. For example, some critics, like in this blog from Columbia University, noted more deaths in this Lancet paper than reported by Australian authorities. Are Australian authorities hiding something, or did Lancet authors fabricated data for their cherished conclusion? I trust Australian authorities more.
A lot of other issues spill around in this crazy dataset, here and elsewhere. Garbage in, garbage out.
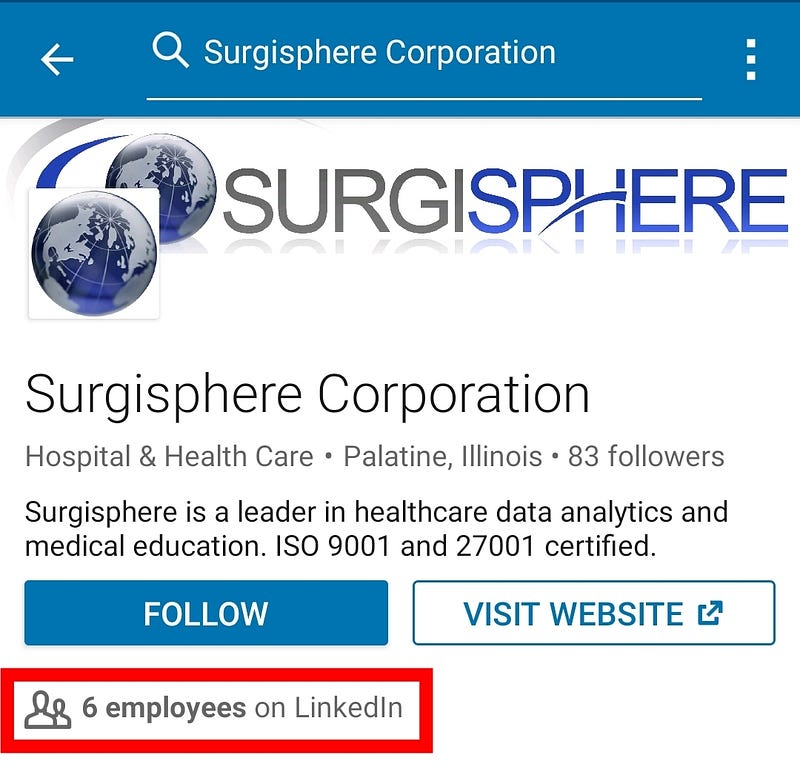
This Lancet paper also has no data traceability. Authors didn’t release the names of people who were responsible for data collection at hospitals. That’s the second black box. The paper only has 4 co-authors (ridiculously small for medical literature standards), and only one person was responsible for data acquisition: Sapan S Desai. How could he curate a huge dataset of 96 032 patients alone, so quickly? Is his company Surgisphere magical? Surgisphere only has 6 employees on LinkedIn. That’s insane.
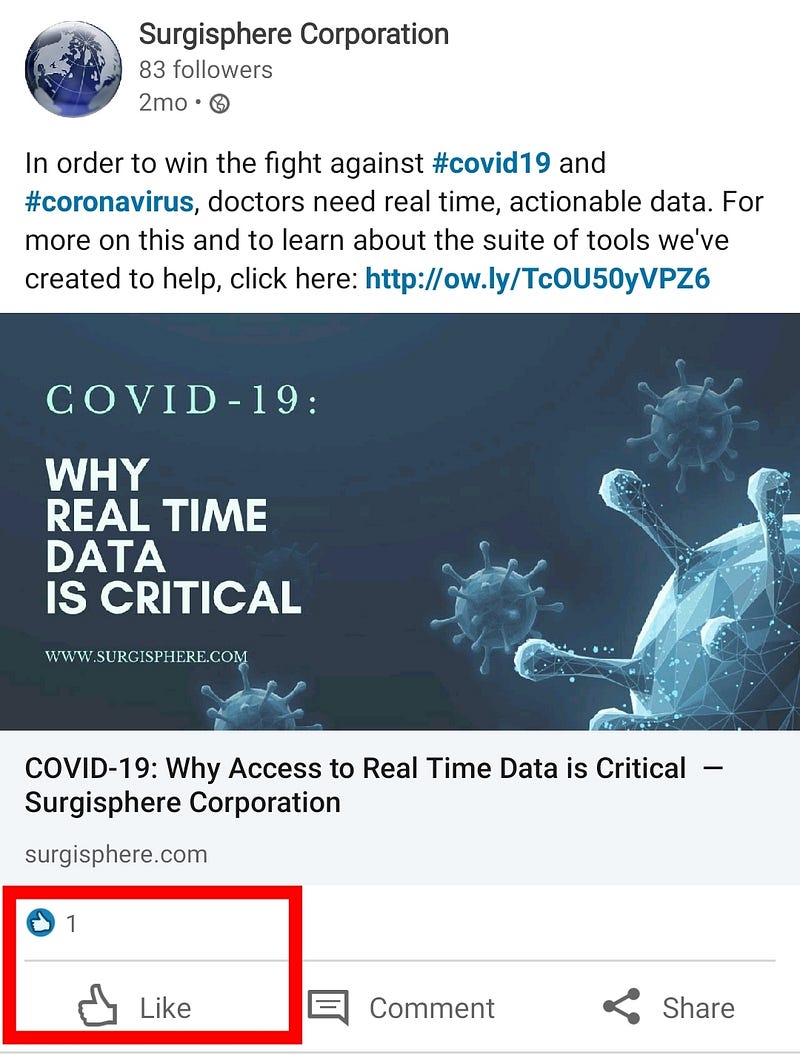
Surgisphere claims to be “a leader in healthcare data analytics”, but their LinkedIn posts only have 1 like (a self-like!), and their oldest post is 2-months old. This bold and shady company seems a bit early-stage for leadership claims. It’s not the Google of health data.
Why didn’t doctors and their labs try to stick their names on this prestigious Lancet paper? So many grants and tenures missed.
As a comparison, in particle physics, the important paper discovering the Higgs Boson particle at the Large Hadron Collider has 5,154 co-authors.

At COVIND data consortium, we are building a network of local doctors, who are also scientific partners. They put their names on all papers where their data is involved. They often tell me that’s their first condition for collaborating. That is not only to acknowledge their scholarly contribution. Their signature is also a public endorsement of the dataset they release, and a key link in the data traceability chain. That’s important when raw identified data is inaccessible for privacy reasons.
Of course, mistakes can happen, we are moving fast, but we implemented transparent error-correction procedures.
Lancet did not release the names of the referees (if any). Yet another black box. So the only signed editorial validation comes from Lancet editor-in-chief Richard Horton.
Richard Horton has an old political conflict with the US President. Donald Trump happens to be a big fan of Chloroquine, and Trump doesn’t care about Randomized Clinical Trials, and about science in general. Horton is certainly tempted to prove him wrong. In this bestial battle of egos, did Horton feel so insecure as to let a fraudulent submission slip through editorial filters? Oups!

Richard Horton published several political rants against Trump in The Lancet:
I don’t trust Fox News about global warming, so why should I trust The Lancet about Trump-approved Chloroquine? Fake news all around.

As written elsewhere in The Lancet (I love it as a political outlet, I am left-wing too!): “public health should not be guided by partisan politics”
That’s true both ways.
As a left-wing citizen, I agree with Richard Horton when he says that this pandemic is “the biggest science policy failure in a generation”, but I am surprised he wanted to be a part of it. So ironic.
From the ashes of the Lancet, I am still hopeful that a new era for data-driven medicine will emerge, based on three pillars: reproducibility, traceability and transparency.
Chris Martenson does a through job analyzing this Lancet study and concluded it is garbage.
Watch is presentation here:
TITLE: Garbage ‘Science’: Be Wary Of What You’re Being Told
https://www.youtube.com/watch?v=IUD_wvkNhnk&feature=youtu.be
Politics, not science.
that link isn’t working for me.
Sorry, the final link in Post # 5 apparently went stale.
But the content of the entire open letter is in Post #5. That’s all I have for now.
HERE IS THE FULL LIST OF SIGNATORIES TO THE LETTER ( I think that’s what you are interested in ):
SOURCE: https://www.facebook.com/avalonwebagency/posts/2948493341900632
This open letter is signed by clinicians, medical researchers, statisticians, and ethicists from across the world.
TITLE: Open letter to The Lancet :
“Your study is irregular and in a blackbox. Transparency is mandatory by the scientific-publication-codes you have signed, and it is needed to verify all the discrepancies of this study.”
LIST OF SIGNATORIES :
Dr James Watson (Statistician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)1
Professor Amanda Adler (Trialist & Clinician, Director of the Diabetes Trials Unit, UK)
Dr Ravi Amaravadi (Researcher, University of Pennsylvania, USA)
Dr Ambrose Agweyu (Medical researcher, KEMRI-Wellcome Trust Research Programme, Kenya) Professor Michael Avidan (Clinician, Washington University in St Louis, USA)
Professor Nicholas Anstey (Clinician, Menzies School of Health Research, Australia)
Dr Yaseen Arabi (Clinician, King Saud Bin Abdulaziz University for Health Sciences, Saudi Arabia)
Dr Elizabeth Ashley (Clinician, Director of the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit, Laos) Professor Kevin Baird (Researcher, Head of the Eijkman-Oxford Clinical Research Unit, Indonesia)
Professor Francois Balloux (Researcher, Director of the UCL Genetics Institute, UK)
Dr Clifford George Banda (Clinician, University of Cape Town, South Africa)
Dr Edwine Barasa (Health economist, KEMRI-Wellcome Trust Research Programme, Kenya)
Professor Karen Barnes (Clinical Pharmacology, University of Cape Town, South Africa)
Professor David Boulware (Researcher & Triallist, University of Minnesota, USA)
Professor Buddha Basnyat (Clinician, Head of the Oxford University Clinical Research Unit - Nepal, Nepal) Professor Philip Bejon (Medical researcher, Director of the KEMRI-Wellcome Trust Research Programme, Kenya) Professor Mohammad Asim Beg (Clinician/Researcher, Aga Khan University, Pakistan)
Professor Emmanuel Bottieau (Clinician, Institute of Tropical Medicine, Antwerp, Belgium)
Dr Sabine Braat (Statistician, University of Melbourne, Australia)
Professor Frank Brunkhorst (Clinician, Jena University Hospital, Germany)
Dr Todd Campbell Lee (Researcher, McGill University, Canada)
Professor Caroline Buckee (Epidemiologist, Harvard TH Chan School of Public Health, USA)
Dr James Callery (Clinician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Professor John Carlin (Statistician, University of Melbourne & Murdoch Children’s Research Institute, Australia) Dr Nomathemba Chandiwana (Research Clinician, University of the Witwatersrand, South Africa)
Dr Arjun Chandna (Clinician, Cambodia Oxford Medical Research Unit, Cambodia)
Professor Phaik Yeong Cheah (Ethicist/Pharmacist, Mahidol Oxford Tropical Medicine Research Unit, Thailand) Professor Allen Cheng (Clinician, Monash University, Australia)
Professor Leonid Churilov (Statistician, University of Melbourne, Australia)
Professor Ben Cooper (Epidemiologist, University of Oxford, UK)
Dr Cintia Cruz (Paediatrician Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Professor Bart Currie (Director, HOT NORTH, Menzies School of Health Research, Australia)
Professor Joshua Davis (Clinician, President of the Australasian Society for Infectious Diseases, Australia)
Dr Jeremy Day (Clinician, Oxford University Clinical Research Unit, Vietnam)
Professor Nicholas Day (Clinician, Director of the Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Dr Hakim-Moulay Dehbi (Statistician, University College London, UK)
Dr Justin Denholm (Clinician, Researcher, Ethicist, Doherty Institute, Australia)
Dr Lennie Derde (Intensivist/Researcher, University Medical Center Utrecht, The Netherlands)
Professor Keertan Dheda (Clinician/Researcher, University of Cape Town, & Groote Schuur Hospital, South Africa) Dr Mehul Dhorda (Clinical Researcher, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Professor Annane Djillali (Dean of the School of Medicine, Simone Veil Université, France)
Professor Arjen Dondorp (Clinician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Dr Joseph Doyle (Clinician, Monash University and Burnet Institute, Australia)
Dr Anthony Etyang (Medical Researcher, KEMRI-Wellcome Trust Research Programme, Kenya)
Dr Caterina Fanello (Epidemiologist, University of Oxford, UK)
Professor Neil Ferguson (Epidemiologist, Imperial College London, UK)
Professor Andrew Forbes (Statistician, Monash University, Melbourne, Australia)
Professor Oumar Gaye (Clinical Researcher, University Cheikh Anta Diop, Senegal)
Dr Ronald Geskus (Head of Statistics at the Oxford University Clinical Research Unit, Vietnam)
Professor Dave Glidden (Biostatistics, University of California, USA)
Professor Azra Ghani (Epidemiologist, Imperial College London, UK)
Prof Philippe Guerin (Medical researcher, University of Oxford, UK)
Dr. Raph Hamers (Clinician/Trialist, Eijkman-Oxford Clinical Research Unit, Indonesia)
Professor Peter Horby (Clinical Researcher, Centre for Tropical Medicine and Global Health, University of Oxford) Dr Jens-Ulrik Jensen (Clinician/Trialist, University of Copenhagen, Denmark)
Dr Hilary Johnstone (Clinical Research Physician, Independent)
Professor Kevin Kain (Clinical Researcher, University of Toronto, Canada)
Dr Sharon Kaur (Ethicist, University of Malaya, Malaysia)
1 For correspondence: james@tropmedres.ac
Dr Evelyne Kestelyn (Head of Clinical Trials, Oxford University Clinical Research Unit, Vietnam)
Dr Tan Le Van (Medical Researcher, Oxford University Clinical Research Unit, Vietnam)
Professor Katherine Lee (Statistician, University of Melbourne, Australia)
Professor Laurence Lovat (Clinical Director of Wellcome EPSRC Centre for Interventional & Surgical Sciences, UCL, UK) Professor Kathryn Maitland (Clinician, Imperial College London/KEMRI Wellcome Trust Programme, Kenya)
Dr Julie Marsh (Statistician, Telethon Kids Institute, Australia)
Professor John Marshall (Clinician/Researcher, University of Toronto, Canada)
Dr Gary Maartens (Clinician, University of Cape Town, South Africa)
Professor Mayfong Mayxay (Clinician/Researcher, Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit, Laos) Dr John McKinnon (Clinician/Researcher, Wayne State University, USA)
Dr Laura Merson (Clinical researcher, University of Oxford, UK)
Dr Alistair McLean (Medical researcher, University of Oxford, UK)
Professor Ramani Moonesinghe (Clinician researcher, University College London, UK)
Professor Bryan McVerry (Medical researcher, University of Pittsburgh, USA)
Professor William Meurer (Clinician/Medical researcher, University of Michigan, USA)
Dr Kerryn Moore (Epidemiologist, London School of Hygiene and Tropical Medicine, UK)
Dr Rephaim Mpofu (Clinician, University of Cape Town, South Africa)
Dr Mavuto Mukaka (Statistician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Dr Srinivas Murthy (Clinical Researcher, University of British Columbia, Canada)
Professor Kim Mulholland (Clinician, London School of Hygiene & Tropical Medicine, UK)
Professor Alistair Nichol (Clinician Researcher, Monash University, Australia)
Professor Francois Nosten (Clinician, Director of the Shoklo Malaria Research Unit, Thailand)
Dr Matthew O’Sullivan (Clinician, Westmead Hospital & University of Sydney, Australia)
Professor Piero Olliaro (Clinical Researcher, University of Oxford, UK)
Professor Marie Onyamboko (Clinical researcher, Kinshasa School of Public Health, DRC)
Dr Marcin Osuchowski (Medical researcher, Ludwig Boltzmann Institute, Austria)
Professor Catherine Orrell (Clinical Pharmacologist, University of Cape Town, South Africa)
Professor Jean Bosco Ouedraogo (Medical Researcher, WWARN, Burkina Faso)
Dr Elaine Pascoe (Statistician, University of Queensland, Australia)
Professor David Paterson (Clinician, Director, UQ Centre for Clinical Research, Australia)
Dr Kajaal Patel (Paediatrician, Cambodia Oxford Medical Research Unit, Cambodia)
Dr Tom Parke (Statistician, Berry Consultants, UK)
Professor Philippe Parola (Researcher, Aix-Marseille University, France)
Professor Paul Newton (Clinician, University Oxford, UK)
Professor David Price (Statistician, Doherty Institute & University of Melbourne, Australia)
Professor Richard Price (Clinician, Menzies School of Health Research, Australia)
Professor Sasithon Pukrittayakamee (Clinician, Mahidol University, Thailand)
Dr Ben Saville (Statistician, Berry Consultants & Vanderbilt University)
Professor Jason Roberts (Pharmacist/Clinician, The University of Queensland, Australia)
Professor Stephen Rogerson (Clinician, University of Melbourne, Australia)
Professor Kathy Rowan (Researcher, Director of the ICNARC Clinical Trials Unit, UK)
Dr William Schilling (Clinician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Dr Anuraj Shankar (Clinician/Trialist, Eijkman-Oxford Clinical Research Unit, Indonesia)
Professor Sanjib Kumar Sharma (Clinician, Koirala Institute of Health Sciences, Nepal)
Professor Julie Simpson (Statistician, University of Melbourne, Australia)
Professor Frank Smithuis (Clinical researcher, Director of the Myanmar Oxford Tropical Research Unit, Myanmar)
Dr Tim Spelman (Statistician, Burnet Institute, Australia)
Dr Kasia Stepniewska (Statistician, University of Oxford, UK)
Dr Nathalie Strub Wourgaft (Clinician, Drugs for Neglected Diseases initiative, Switzerland)
Dr Aimee Taylor (Statistician, Harvard T.H. Chan School of Public Health, USA)
Dr Walter Taylor (Clinician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Professor Guy Thwaites (Clinician, Director of the Oxford University Clinical Research Unit, Vietnam)
Professor Tran Tinh Hien (Clinician, Oxford Clinical Research Unit, Vietnam)
Professor Steven Tong (Clinician, University of Melbourne, Australia)
Professor Paul Turner (Clinician/Researcher, Director of Cambodia Oxford Medical Research Unit, Cambodia) Professor Ross Upshur (Head of Division of Clinical Public Health, University of Toronto, Canada)
Professor Rogier van Doorn (Clinical Microbiologist, University of Oxford, UK)
Professor Sir Nicholas White (Clinician, Mahidol Oxford Tropical Medicine Research Unit, Thailand)
Professor Thomas Williams (Clinician, KEMRI-Wellcome Trust Research Programme, Kenya)
Professor Chris Woods (Researcher, Duke University, USA)
Dr Sophie Yacoub (Clinician, Oxford University Clinical Research Unit, Vietnam)
Professor Marcus Zervos (Researcher, Wayne State University School of Medicine, USA)
Believe the Science.
Obey.
more evidence Richard Horton is a lunatic:
15 Oct 2019: Offline: Extinction or rebellion?
by Richard Horton
An unprecedented social and political disruption is about to take place. Nothing like it will have been seen for a generation. The outcome could be transformational. Or it could be met with indifference. Remember the antiglobalisation protests in Genoa in 2001? Or the marches against the Iraq war in 2003? The outcomes of October, 2019, depend on the intensity of resistance.
What part should health workers play in one of the greatest social movements of our time? The occasion before us is 2 weeks of non-violent direct political action, beginning on October 7. Billed as an “International Rebellion”, millions of people will gather in cities around the world. They will “continue to rebel against the world’s governments for their criminal inaction on the climate and ecological crisis”. Citizens are encouraged to take as much time off work as possible...
Extinction Rebellion puts it like this: “Because time is running out. We’re almost at the point of no return. The governments are doing nothing. Businesses are doing nothing. The situation is urgent.” There are three demands—first, tell the truth; second, act now; third, go beyond politics to create a citizens’ assembly. A citizens’ assembly will reclaim control from a paralysed political process. To challenge corporate lobbyists. To explore the complexity of our shared predicaments. And to agree solutions. In sum, to break the political deadlock...
The climate crisis is one of the greatest threats to the health of humanity today...
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)32260-3/fulltext
China acted “TREMENDOUSLY DECISIVELY”...
Youtube: 5m48s: 1 May: CGTN: Richard Horton/Lancet: Richard Horton: China’s reaction is decisive and quick
Richard Horton, editor-in-chief of the prominent British medical journal The Lancet, said in an interview with CCTV on Friday that China’s reaction is decisive and quick, including the decision on the lockdown of Wuhan...
https://www.youtube.com/watch?v=nQ61w_lIacQ
Youtube: 3m19s: 24 Oct 2019: Health and Climate | Richard Horton/Lancet
‘All health professionals have a duty and obligation to engage in all kinds of non-violent social protest to address the climate emergency’ - Richard Horton
Richard Horton of The Lancet discusses why the climate emergency is “the most existential crisis facing our communities in the world today” and why taking action is “the duty of a doctor”.
https://www.youtube.com/watch?v=YEVGNeneYug
They expected “integrity”? Ha ha. Good one.
Did anyone catch the absurdity of this?
The whole study is fraudulent.
It reports by race in France when France by law never gathers such data.
Australian hospitals mentioned actually say they never were contacted for the data that study pretends to have. Bonus, it reports out of 5 hospitals in hundreds 73 deaths when there were on 67 nationwide. Authors say they accidentally included an asian hospital.
Also impossible is their ratio of smokers being prettry much completely even across all nations. They show obvious doctoring around 10%
Indeed, if they cannot explain where they get their data, then scientifically it is worthless..
Lack of transparence? It is more than that, it is fiction!
Climate Change bull sht the same.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.