JOHN SANFORD’S GENETIC ENTROPY AND THE MYSTERY OF THE GENOME
The errors in Genetic Entropy [24]are so pervasive that it might take a whole new book to fully expose them [93]. I’ll break it down to the topics listed below:
(1) Kimura’s Distribution of Mutations
(2) Evidence for Beneficial Mutations
(3) Gene Duplication
(4) Natural Selection: What Sanford Claims
(5) Natural Selection: What Studies Show
(6) Evidence for Genomic Deterioration
(7) Synergistic Epistasis and Other Theoretical Considerations
(8) Genetic Entropy and the Mystery of John Sanford
These topics deal with the two broad areas of random mutations and natural selection. These are the twin pillars of the neo-Darwinian synthesis, which Sanford refers to as the Primary Axiom.
(1) Kimura’s Distribution of Mutations
Sanford makes unsubstantiated claims that the number of beneficial mutations is insufficient to account for the increase in genomic complexity demanded by evolutionary theory in going from single-celled organisms to today’s mammals, or to compensate for the effects of deleterious mutations. He attempts to justify these claims in two main ways: (a) by appealing to a graph by Motoo Kimura, and (b) by citing some bacterial studies which show low rates of beneficial mutations. Kimura’s plot is discussed in this section, and the bacterial studies are treated in the following section.
Sanford presents a graph (Figure 3c), “modified and expanded” from the work of Motoo Kimura [25], which Sanford claims “very nearly represents the true distribution of mutations.“ The x-axis represents the severity of the effect of the mutations, and the vertical distance denotes the relative number of mutations with a given effect.
The curve for deleterious mutations is in the upper left quadrant. It starts near the x-axis at the extreme left (negative values of mutation effect), and rises steeply as it approaches the y-axis. It appears to asymptote to both the x- and the y-axes. It indicates that most mutations are harmful, and that mutations of small effect are more common. Sanford shows a zone of “near-neutrality” on either side of the y-axis. This is where the effect of a mutation is so subtle that it cannot be effectively selected for or against. Mutations in this zone can be regarded as “near-neutral” or “effectively neutral.” Kimura has shown that the width of this zone of near-neutrality gets smaller as the size of the effective breeding population, Ne, gets larger.
Sanford (pp.22-23) professes puzzlement over Kimura’s omission of beneficial mutations:
In Kimura’s figure, he does not show any mutations to the right of zero – i.e. there are zero beneficial mutations shown. He obviously considered beneficial mutations so rare as to be outside of consideration….…Given the pivotal role beneficial mutations play in all evolutionary scenarios, I was puzzled as to why Kimura did not represent them in any way in his graph.
Sanford notes, fairly enough, that beneficial mutations are much less common than deleterious mutations, and so he draws a little wedge-shaped curve on the right-hand side of the graph (Figure 3d) to represent beneficial mutations on the same scale.
In this exercise, Sanford (p.24) is “shocked” to find that that very few beneficial mutations are of large enough effect to be selected for:
What is most interesting about this figure (it came as a shock to me) is to realize that essentially the entire range of all hypothetical beneficial mutations falls within Kimura’s “effectively neutral” zone. That means that essentially all beneficial mutations (to the extent that they actually happen), must be “un-selectable.” So selection could never favor any such beneficial mutations, and they would essentially all drift out of the population. No wonder that Kimura preferred not to represent the distribution of the favorable mutations!
In these passages, Sanford would have his readers believe that Kimura was so dismayed by the evolutionary implications of having only small numbers of beneficial mutations that Kimura did not dare treat the subject. That is an inexcusable misrepresentation of the facts. Kimura [26] tells us explicitly why he omitted beneficial mutations from this model, and it has nothing to do with how rare or unselectable they are:
In this formulation, we disregard beneficial mutations, and restrict our consideration only to deleterious and neutral mutations. Admittedly this is an oversimplification, but as I shall show later, a model assuming that beneficial mutations also arise at a constant rate independent of environmental changes leads to unrealistic results.
The “unrealistic results” that Kimura notes of plugging beneficial mutations into his model are that “the rate of evolution can become enormously high in a very large population” (i.e. beneficial mutations would become fixed at high rates), which is not an effect that is normally observed in reality. So the reason Kimura omitted beneficial mutations is not that they have too little effect (as Sanford implies), but that in his model they would havetoo much effect. This is just an artifact of his model (which, like all models, is a simplification of the real situation), not a statement about whether beneficial mutations have an effect in the real world.
Kimura [27] acknowledges that with his model, slightly deleterious mutations can build up, but (using parameter values he considers realistic), he does not see this as a threat to most species:
Under the present model, effectively neutral, but, in fact, very slightly deleterious mutants accumulate continuously in every species. The selective disadvantage of such mutants (in terms of an individual’s survival and reproduction – i.e. in Darwinian fitness) is likely to be of the order of 10-5 or less, but with 104 loci per genome coding for various proteins and each accumulating the mutants at the rate of 10-6 per generation, the rate of loss of fitness per generation may amount of 10-7 per generation. Whether such a small rate of deterioration in fitness constitutes a threat to the survival and welfare of the species (not to the individual) is a moot point, but this can easily be taken care of by adaptive gene substitutions that must occur from time to time, say once every few hundred generations.
In other words, within his theoretical framework, the mutational load can be readily compensated by the occasional beneficial mutation.
All this is clearly spelled out by Kimura in The Neutral Theory of Molecular Evolution,which Sanford cites in his Genetic Entropy. Thus, Sanford has elected to mislead his readers on Kimura’s treatment of beneficial mutations, knowing that few of them will dig into Kimura’s writings to expose the deception.
***************************************************
Sanford notes that Kimura’s curve does not show any mutations with exactly zero effect, i.e. as absolutely neutral, and claims that, since (according to this curve) essentially all mutations are deleterious, and many of those deleterious mutations are too small to select against, genomes must accumulate ever-growing numbers of deleterious mutations which degrade the information of the genome. Thus (p.25), “Figure 3d vividly illustrates why mutations cannot result in a net gain of information…Everything about the true distribution of mutations argues against their possible role in forward evolution.”
Since this curve seems central to Sanford’s thesis, it bears some scrutiny. Here is what Kimura [28] says about the genesis of the left-hand (deleterious mutations) curve:
In our discussion of the relationship between the rate of evolution and selective constraint (see chapter 7), we often found it convenient to classify mutations into two distinct types, strictly neutral and definitely deleterious, and argued that the proportion of neutral mutations decreases with increasing functional constraint. In reality, there may be a continuum between these two types and the possibility cannot be excluded that the intermediate types between these two extremes are important. In other words, at the molecular level, a substantial proportion of new mutations could be very slightly deleterious as emphasized by Ohta (1973, 1974). Such mutations behave as if selectively neutral in a small population, but are unequivocally selected against in a large population….. I proposed (Kimura, 1979) a model which assumes that the selection coefficients among mutants follows a Gamma ( Γ) distribution and this was termed the model of effectively neutral mutations. This model is based on the idea that selective neutrality is the limit when the selective disadvantage becomes indefinitely small.
There is no experimental data of mutation frequency versus fitness effect plotted to verify the accuracy of this curve. It is not based on any actual data. It is merely an abstract relation, chosen for convenient mathematical manipulation and to illustrate a general point. It does not mean that Kimura really believes there are no truly neutral mutations; a cursory reading of his work shows that the concept of neutral mutations remains at its core. Nobody really knows the fitness distribution for mutations in humans, which is what Sanford is most concerned with, so it is inappropriate to draw hard conclusions from this curve.
**************************************************
One final distinction — as noted above, Sanford (p. 24) states that:
Essentially the entire range of all hypothetical beneficial mutations falls within Kimura’s “effectively neutral” zone. That means that essentially all beneficial mutations (to the extent that they actually happen), must be “un-selectable”.
The key, slippery word here is “essentially.” There is a world of difference between “rare” and “absent” as descriptors of beneficial mutations. Sanford makes the classic creationist leap from “the vast majority of mutations have little or no beneficial impact” to “effectively all mutations have little or no beneficial impact.” But that position is falsified by the many studies that show that organisms do in fact adapt to environmental changes by mutation and natural selection. Thus, in reality there is a small but non-negligible tail on the beneficial mutation distribution which extends far enough to the right on this type of plot to represent the beneficial mutations which are selected for.
(2) Evidence for Beneficial Mutations
The literature is rife with examples of helpful new mutations becoming fixed in a population which is exposed to a new environment. Since the fitness of an organism can only be defined in a particular environment, these mutations must be considered as beneficial for those populations. There is no necessity in evolutionary theory for a mutation which is helpful in the new environment to also be useful in the old environment. Nevertheless, there are examples where mutations caused an organism to be become more fit in a new environment, while remaining competitive in the original environment. A number of these examples were listed above [4-9]. These cases directly refute the notion that essentially all mutations are degenerative.
For most organisms, if you put them in a new environment so you can detect adaptive mutations, and wait some hundreds or thousands of generations with a population of over say 1000, you will find that adaptive, i.e. beneficial; mutations have occurred and become fixed. The appearance and fixation of beneficial mutations in situations like this is not scarce or tenuous; rather, it is common and reliable. Therefore, it is obviously wrong for creationists like Sanford to claim that beneficial mutations are so rare that they cannot be effective in producing adaptive changes.
Sanford (p. 24) mentions three studies to buttress his erroneous claim:
I have seen estimates of the ratio of deleterious-to-beneficial mutations which range from one thousand to one, up to one million to one. The best estimates seem to be one million to one (Gerrish and Lenski, 1998). The actual rate of beneficial mutations is so extremely low as to thwart any actual measurement (Bataillon, 2000; Elena et al.,1998).
We will examine these studies and their contexts to expose Sanford’s misrepresentations. First, Gerrish and Lenski’s [29] 1:1,000,000 estimate was for the ratio of beneficial to total mutations, not for beneficial to deleterious mutations. Although they do not directly state the nature of the 999,999 non-beneficial mutations, there is no reason to assume that they are all or mostly deleterious.
More importantly, consider the experimental data from which Gerrish and Lenski draw their conclusions: Lenski’s well-known long-term E. coli evolution study. His group set up twelve populations of E. coli, starting from a single clone progenitor, and let them evolve under nutrient-limited conditions. [30] This study was described in my July letter [“STAN 3”]. The effective breeding size of each population was about 3.3 x 107 [29].
One beneficial mutation in a million sounds like a very low number, but with thousands of generations and millions of individuals in the breeding population, there were many beneficial mutations which available in the Lenski experiment for natural selection to work with. Gerrish and Lenski [29] estimate that a new beneficial mutation occurred about every fifteen generations in their experimental populations. About 1% of the estimated beneficial mutations actually got fixed in the population. [31] For larger organisms, the breeding populations are typically lower, but the number of mutations per individual per generation is much higher, due to larger genomes and higher mutation rate per DNA base pair, so there are still lots of mutations available. However, for both microbes and mammals, evolution is typically a relatively slow process, so hundreds or thousands of generations may be required to effect a discernable change in the breeding population.
For twelve out of twelve populations in the Lenski study, a significant increase in fitness was observed over the first 10,000 generations. Thus, whatever was the percentage of beneficial mutations, they were numerous and effective enough to drive the evolution of each of the experimental populations to improved fitness. This completely refutes Sanford’s claim that “effectively all beneficial mutations…must be un-selectable.” Sanford had to be aware of the Lenski long-term E. coli study, since it was the basis of Gerrish and Lenski’s estimates of beneficial mutations, yet he withheld that information from his readers and made statements about beneficial mutations that are obviously false.
The 1998 Elena et al. study that Sanford cites also comes from Lenski’s group. Elena et al. [32] took an E. coli cell from one of the populations which had been evolved for 10,000 generations, and constructed 226 artificial mutants. Each mutant contained a single random insertion of one of three transposons. Each of these transposons encoded resistance to one of three different antibiotics. A phage was used as the delivery vehicle. These mutants were assayed for fitness relative to a common competitor. None of the mutations had a significant positive effect on fitness in their standard glucose-limited environment, whereas 80% had a significant negative effect (average 3% fitness reduction).
It is crucial to note that the starting cell had already been heavily adapted to the particular test environment for 10,000 generations. By this point, the rate of fitness increase in the population had slowed to a crawl [33]. This means that this population was so well adapted to its environment that the millions of naturally-occurring mutations in the population were conferring little further benefit. Therefore we would expect that that these mere 226 additional mutations would show no further beneficial effects, and that they were in fact likely to push it away from its local optimum. Moreover, insertion mutations of this type are likely to block the formation of any functional gene product [34]. Sanford withholds from the reader the fact that this study of artificial mutants was derived from the Lenski long-term E. coli project, which showed unequivocally that beneficial mutations are numerous and effective enough to be naturally selected and to improve the fitness of populations.
Sanford further withholds a paper by Remold and Lenski [35], which was a follow-up study to the Elena et al. [32] paper, and which demonstrated a relatively high proportion of beneficial mutations when a different environment was considered. Out of the 226 mutants from Elena et al. [32], Remold and Lenski [35] randomly chose 9 mutants from each of the three antibiotic resistance classes. Of these 27 strains, one was eliminated due to contamination. The remaining 26 strains were evaluated for fitness in 4 different assay environments: glucose medium at 28 C and at 37 C, and maltose medium at 28 C and at 37 C.
In the original glucose nutrient medium, to which the parent cell (prior to the insertions of the single mutations) had been adapted for 10,000 generations, the mutants showed fitness within about 2% percent of the progenitor. Thus, as in the original Elena et al. [32] study, no significant beneficial effects were seen from the mutations when the bacteria were tested in the glucose medium. However, Remold and Lenski [35] saw much different results in the maltose medium. The maltose medium represents a different environment, to which the parent cell had not already been adapted. In this case, there was a much wider spread of fitness. In maltose, most of the 26 mutations were deleterious, but at least 3 (i.e. 12% of the total 26 mutations represented) were significantly beneficial. So here we have a study showing 12% of mutations being beneficial, but Sanford’s readers are told nothing of this.
I was unable to access the Bataillon 2000 study [36] that Sanford cites, but a more recent publication from Bataillon gives a much different perspective on beneficial mutations than the one Sanford presents. Kassen and Bataillon (2006) [5] took a wild-type Pseudomonas flourescens bacterium, and exposed it to an antibiotic. They obtained over 600 antibiotic-resistant strains, with an estimated frequency of 2.4 x 10-9 beneficial mutations per cell division. That seems like a tiny number, yet it was adequate to drive the evolution of fitter bacteria. These antibiotic-resistant strains were much fitter in the new environment than the parent wild-type bacteria, which could not survive at all in the presence of the antibiotic. Interestingly, even in the absence of antibiotic, at least 2.7% of the mutants were superior to the wild-type. This is just one of the examples we have mentioned where mutant organisms can be superior to the parent in both the new environment and in the original environment.
This Kassen and Bataillon study was not available when Sanford wrote Genetic Entropy. Neither were studies by Perfeito et al. [37] which found that 1 out of every 150 mutations were beneficial in small populations of E. coli, or by Joseph and Hall [38] who found 13% of the mutations in yeast were beneficial.
However, the 2001 study by Imhof and Schlotterer [39] was accessible to Sanford at the time. Imhof and Schlotterer estimated the rate of (fixed) beneficial mutations to be 4 x 10-9 per cell per generation for cultures of E. coli, similar to the later Kassen and Bataillon study. Again, this seems like a low number, but in evolution there are many organisms and many generations, so the net amount of beneficial mutations is appreciable and effective. It is certainly not “so extremely low as to thwart any actual measurement.” Again, Sanford does not expose his readers to this inconvenient truth.
(3) Gene Duplication
Sanford repeatedly asserts that mutations, by which he seems to mean simple point substitutions or single point insertion/deletion events, do not increase net information. That is generally true for point substitutions or indels, but irrelevant. By “increase net information” I assume Sanford means “increase size of the functional genome” or “increase the number of distinct genes.” This obviously will not be accomplished by just substituting one amino acid for another at a given point.
However, there is a whole other class of mutations which are common and which do increase genomic size. These are duplications and insertions of genetic material, ranging from small chunks of DNA to complete genes and to duplication of entire genomes. As usual with major mutations, most of these duplication/insertion events will be deleterious to the organism, but a small fraction will be beneficial, and some will be effectively neutral. In my letter of July [“STAN 3”] I cited three studies showing beneficial gene duplications [9, 40, 41]. Gene duplication followed by further, normal mutations provides a clear path to increasing genomic complexity. Creationists are unable to demonstrate that this path is not viable. This rebuts their claim that natural causes are inadequate to account for the increase in genomic complexity in the evolution of vertebrates from simpler organisms.
In some cases the duplicated genes are different from the parent gene, so some variation is introduced right away [9,90,91]. I am not aware of a study which has followed the genetic path of an organism through gene duplication and a subsequent major refunctionalization, but I would not expect that to be readily observable. Evolution is a very slow process by human standards. Beneficial gene duplication is rare, and the modification of a gene to serve a new function is rare, so the odds of observing both these events for a single gene in the span of a human lifetime, in an organism that happens to be under observation, are low indeed. However, in the wild, with trillions of organisms and hundreds of millions of years, the odds lengthen for successful gene duplication with neofunctionalization. The review by Long et al. [90] cited above notes a number of inferred examples of neofunctionalization of duplicated genes, but many of these transformations spanned millions of years.
Another window into the rarity of fixed mutations which increase complexity comes from examination of changes in physical forms (phenotypes) in the fossil record [44]:
Production of man from a primitive jawless fish in half a billion years is a remarkable example of progressive evolution, but we should not forget that degeneration and extinction are much more common in evolution. Haldane (1958) calls attention to the fact that probably for every case of progressive evolution in the sense of descendants being more complex in structure and behavior than their ancestors, there have been ten of regressive evolution. The main reason that evolution as a whole appears to be progressive is simply because a species that acquired a new capacity was more likely to give rise to various descendant species than one which lost some capacities.
This balance between genetic advance and decay does not imply the absence of complexity-increasing mutations, but does suggest that they are so rare that we should not expect see them occur under human observation.
Sanford tries to dismiss gene duplication with ridicule, not logic. He makes up some examples of nonsense duplications of letters and words that are not germane to genomes. He does not seem to understand that the genome is like a recipe, not a descriptive essay. Repeating a sentence in an essay may not add any information, but duplicating an instruction in a cake recipe will give a different cake. Thus, changing a recipe segment from “Add 1 cup flour” to “Add 1 cup flour; Add 1 cup flour” can materially affect the product. A further mutation to one of the duplicate instructions could give “Add 1 cup flour; Add 1 cup pudding,” which clearly contains more information that the original “Add 1 cup flour.” Of course, we would not expect these recipe permutations to survive and be recorded and shared unless the new cakes had some attractive characteristic. This would be natural selection in action.
(4) Natural Selection: What Sanford Claims
In evolutionary theory, the variations among individuals due to differing genetic endowments can lead to differing success in survival and reproduction. Thus, the genes of the fitter individuals become more highly represented in the population.
It is common for creationists to claim (incorrectly) that there are no or effectively no beneficial mutations, or none that contribute to increasing genome complexity. However, most of them seem to accept the efficacy of natural selection. A distinctive of Sanford’s work is his claim that, even if there were significant beneficial mutations, natural selection does not have the power to implement them, or to keep deleterious mutations from accumulating.
Sanford attempts to build a case that the effectiveness of natural selection is too limited to prevent the deterioration of genomes, especially human genomes. He mentions a number of theoretical or potential constraints on selection, such as:
(a) Nature sees the whole organism, so individual nucleotides cannot be selected for or against (p. 47).
(b) There are many levels of biochemical organization standing between a nucleotide and the whole organism (p. 48).
(c) Each nucleotide and gene exists in a cluster of many other nucleotides and genes (p. 54).
(d) There is a cost to selection – - some fraction of the population must be removed each generation (p. 57).
(e) Most deleterious mutations are so subtle that they do not produce an effect which is recognizable, at least to a human observer, and thus they will be difficult to select against (p. 61).
(f) Some theorists claim that it is not possible to select for many traits simultaneously (p. 78).
(g) Selection for one trait may interfere with selection for another trait, especially if the genes for the two traits are physically linked (p. 79-82).
(h) Various types of “noise” can interfere with the heritability of a trait, e.g. when an individual’s reproductive success is affected by factors other than genetic endowment (pp. 89-99).
He notes that natural selection should work more effectively with microbes, since these tend to have simpler genomes, large breeding populations, and more direct effect of selection on the individual cell. This contrasts to mammals, which typically have large genomes, more genomic interactions, and small populations which cannot tolerate a lot of selection cost (p. 74).
These factors (a)-(h) can indeed diminish the effectiveness of natural selection to some extent. Sanford, however, makes a giant and unwarranted leap to declare that they negate the broad effectiveness of natural selection. Sanford concedes that natural selection works at some limited level, i.e. that it can shape certain gene frequencies (p.64), but claims that it cannot protect higher genomes against deterioration (p. 83):
While selection is essential for slowing down degeneration, no form of selection can actually halt it….The extinction of the human genome appears just as certain and deterministic as the extinction of the stars, the death of organisms, and the heat death of the universe.
Sanford acknowledges that selective breeding can be used to promote a particular single trait such as increased seed yield in a plant, but denies that it can halt the overall degradation of genomes due to the accumulation of deleterious mutations. This claim is the core thesis of his book. It is obviously wrong, as will be shown in the next section.
(5) Natural Selection: What Studies Show
Stability of Microbial Genomes
If all the problems ((a)-(h) above) with natural selection were as dire as Sanford claims, even microbial populations should be subject to mutational meltdown, though perhaps at a lower rate per generation than other life-forms. Microbial life-spans can be days, rather than decades. If the buildup of deleterious mutations were a real phenomenon, it would become apparent over thousands of generations in laboratory flasks of bacteria. Indeed, asexual populations like bacteria should be even more vulnerable to the buildup of deleterious mutations, since they would have less opportunity to reshuffle genes to help weed out the bad ones.
If Sanford’s thesis were correct, we might expect an initial bump up in fitness as the bacteria adapted to some new conditions, followed by a reversion to a secular decline in fitness as the supposedly relentless accumulation of deleterious mutations proceeded. In fact, the Lenski long-term E. coli experiment shows the contrary: an initially rapid increase in fitness, followed by a long plateau of stable or slightly increasing fitness for over 35,000 generations [46]. This demonstrates that Sanford is wrong. We note that the populations of cells in Lenski’s flasks are not large by microbial standards – - only about 30 million each, which compares to today’s human populations in many countries.
As for organisms in the wild, I am not aware of evidence for a general decline in microbial fitness over the past decades or millennia, which represent millions of generations. The opposite trend is suggested by how hard we must work to fend off the microbes which afflict our crops and ourselves.
Mutation Accumulation Experiments With Eukaryotes
Multi-celled eukaryotes such as animals have more complex genomes and typically smaller breeding populations than bacteria. Mutation accumulation studies have been made with animals, especially with small insects which are cheap to maintain and have short generational times. In these studies, a line or lines of descent are maintained in which natural selection is eliminated. One way to accomplish this is by having a human researcher randomly select one individual or breeding pair from each generation to produce the next generation. Whatever mutations occur in each generation are retained, since there is no opportunity to weed them out.
Classic mutation accumulation (MA) experiments were done on the fruitfly Drosophila melanogaster by Mukai [46, 47] and Ohnishi [48] in the 1960s and early 1970s. In these experiments, replicated second chromosomes were allowed to accumulate mutations, sheltered from selection by being transmitted only through heterozygous males. The egg-to-adult viability declined by 1%-2% per generation. Various assumptions were employed to analyze the data from these experiments using balancer chromosomes. The results were interpreted to mean that each newborn fly (diploid genome) harbored roughly one new moderately deleterious mutation, with average effects on viability of 1-2%, or 5-10% if homozygous. This is a relatively high load of deleterious mutations, which raised questions on how populations could maintain themselves.
MA experiments are tedious and time-consuming – Mukai’s experiments involved millions of flies. For decades there was little further work in this area, and Mukai’s results (implying a high rate of mildly deleterious mutations) were accepted as truisms, applicable to organisms in general. In the late 1990’s, however, further MA studies were undertaken which gave much different results. Experiments with Drosophila by Fernandez and Lopez-Fanjul [49-51] showed deleterious mutation rates an order of magnitude lower than Mukai, with larger effects per mutation. Keightley and Caballero [52] found almost no decline in fitness over 60 generations in MA experiments with the nematode C. elegans; the estimated rate of deleterious mutations was two orders of magnitude lower than the Mukai results. Fry, et al. [53] performed MA experiments on Drosophila with a procedure similar to Mukai, but found the decline in viability and incidence of mildly deleterious mutations was much smaller. On the other hand, Bryant and Reed [54] saw rapid decline in late-life fecundity in a MA experiment with the housefly.
Keightley and Caballero [52] and Fry, et al. [53] critiqued Mukai’s procedure and analysis, noting some internal contradictions. It is of course possible that Mukai’s and Oshniki’s work was flawed and should be set aside. However, a different perspective is provided by Baer et al. [55]. They suggest that all of the above results may be correct, simply reflecting a wide variation of mutation rates from population to population. In their own experiments, Baer et al. found significant variation in mutation rates among species of nematodes and even between strains within a species.
Another study showing variations of fitness declines between similar populations comes from Lomnicki and Jasienski [56]. Two populations of flour beetles (Tribolium confusum) were maintained for 22 generations in the virtual absence of selection. The starvation resistance of adult beetles from both selection-free populations was reduced by more than 2% per generation, while neither population showed a change in larva-to-adult survivorship rates. However, in one of these populations, the beetles showed slower development and smaller sizes of females, whereas these fitness traits were not eroded in the other population.
Lynch et al. [57] provide a similar perspective. They compare their detailed genomic analysis of yeast from a MA experiment by Dickinson over 4800 generations [34] with literature results for other organisms, and note significant differences among species. Drosophila seems to have an unusually high rate of deleterious mutations compared to other species.
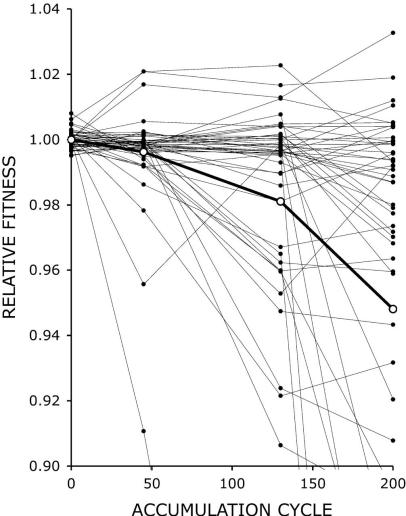
In Dickinson’s experiment [34], 48 separate lines of yeast were maintained for 4800 generations. Fitness was assayed at the start and at three more points in the experiment. Though there was a clear trend on average towards reduced fitness, there was a wide variety in the responses of the individual lines, as shown below in Figure 1. The heavy line shows the average (mean) fitness of all 48 lines at each evaluation point. For any experiment that tries to quantitate fitness, some measurable quantity must be chosen as an estimator of fitness. Here, growth rates of samples from the lines, compared to the parent, were used to estimate fitness. Most lines showed little change in fitness, some showed dramatic decline, and a few actually showed slight fitness improvement. It seems that for any given line, a particular mutation may occur which can cause the fitness of that line to change at some atypically high or low rate. This contingency effect was seen in the Lenski long term E. coli experiment, where some particular, apparently neutral (by itself) mutation occurred early in the history in one out of twelve populations [33]. That pre-adaptive mutation enabled that population to make a jump upward in fitness many generations later.
Figure 1, taken from Dickinson [34] (Copyright © 2008 by the Genetics Society of America )
Another factor is that environmental conditions may impact the genetic fitness of a population. Shabalina et al. [58] ran an MA experiment with two populations of Drosophila kept under different conditions. The number of surviving offspring per female declined by 0.2% per generation for benign competitive conditions, and 2.0% for harsh conditions. On the other hand, Baer et al. [59] ran MA experiments with various strains of nematodes at 20 C and 25 C, and found little evidence that environment affected mutational properties.
Avila et al. [60] ran a MA experiment with Drosophila, where three large control populations were kept to compare with the MA lines. Two on the controls were kept at the normal 25 C temperature, while one was kept at 16 C. The intent with the lower temperature control was to slow down time between birth and reproduction, to produce fewer generations and thus (in theory) give even less chance for genetic change across the duration of the experiment. Surprisingly, the fitness of the second chromosomes from this control showed drastically reduced viability. This population was then brought to 25 C, and fitness recovered almost entirely after ten generations. These results show both the potential for environment to affect fitness and the power of natural selection to restore a deteriorated genome.
This survey of MA experiments reveals a rich set of phenomena. However, the following general trends are clear: in MA experiments, fitness declines at some noticeable rate (e.g. 0.1-1% per generation), whereas fitness does not decline when there is a large enough breeding population (e.g. over 2000 for eukaryotes [61] ) and conditions where differential survival or mating takes place. One can find occasional exceptions to this rule, but it holds in the vast majority of cases. This can be restated as: when natural selection is not operating, the population genome deteriorates, and when natural selection is operating, the average genome of the population does not deteriorate.
This directly contradicts Sanford’s claim that natural selection is ineffective at preventing deterioration of the genome. The factors (a)-(h) listed above are not sufficient to break the connection between gene frequencies in a population and the reproductive success of individuals carrying those genes. Natural selection is effective at weeding out deleterious mutations, despite the impacts of gene linkage, cost of selection, and noise.
This is so simple and so obvious, that we must ask: How can Sanford possibly claim what he claims, when decades of experimental studies clearly show the exact opposite? As a genetics researcher, he was certainly familiar with MA studies and their implications. This is another example of deceit in Genetic Entropy, and it is a whopper.
Fitness Recovery Experiments
The power of natural selection is further demonstrated in fitness recovery experiments, where a line where the genome has deteriorated is then bred under conditions where random mutations and natural selection can shape the gene frequencies. These conditions include a large population (to give variety of genes to select among, and to get greater opportunity for new mutations to arise) and competitive survival/breeding conditions. In the study of Avila et al. [60] noted above, a population of Drosophila had its fitness partly restored after four generations, and almost entirely restored after ten generations of breeding under more favorable conditions.
Fitness recovery experiments after a MA experiment represent a particularly challenging assignment for evolution: for these deteriorated lines, the original population genetic diversity has been lost since the breeding line in an MA experiment is typically narrowed down to just a single individual or breeding pair, and deleterious mutations have accumulated. Estes and Lynch [62] ran fitness recovery tests on MA lines of the nematode Caenorhabditis elegans, which had previously propagated as single individuals for each generation and which had suffered noticeable declines in fitness. These lines were then maintained in several large populations under competitive conditions, to allow the workings of mutations and natural selection. Progeny production and survival to maturity were assayed after 10 and after 80 recovery generations. After 10 generations, there was significant recovery of average fitness, and by 80 generations fitness recovery was nearly complete, as averaged across all the recovery populations. There was significant variation in fitness recovery from population to population, reminiscent of the variations seen among the individual lines of yeast in Dickinson’s MA study [34]. Whitlock et al. [63] cite several additional studies of fitness increase and fitness recovery in viruses and bacteria, which demonstrate the efficacy of mutations and natural selection to improve the genome.
Humans carry around 100-200 new mutations per generation [94]. That seems like a lot, but that is only about one mutation in each 15 to 30 million nucleotides. While a few of these mutations can have devastating health effects, most of these mutations have no apparent physical impact. Most of these mutations fall in regions of the genome with no known function, and many mutations in protein coding regions are “silent” mutations which do not alter the protein which is ultimately formed. Even within the coding for proteins, many of amino acids can be altered without appreciable harm to the individual [95].
The fossil record allows us to trace back some lineages for millions of years. Fossils are found in sedimentary rock layers, which record a succession of biological forms. Radioactive dating of igneous rocks above and below these sedimentary layers provide absolute dating of the sedimentary layers and thus of the fossils within them. Most species and genera go extinct and are replaced by others. However, a few have endured with little alteration for tens of millions of years, representing millions of generations. For instance, both the opossum and the crocodile have been largely unchanged for more than 60 million years. This is further evidence that genomes can endure without rapid deterioration.
As a sidebar, I am aware that creationists claim that radioactive dating of rocks is unreliable, and so these “millions of years” ages for fossils cannot be taken seriously. The creationist case against radioactive dating is based on ignorance and sloppy technique, as noted in my May letter of last year [“STAN 2”].
(6) Evidence for Genomic Deterioration
We have seen that there is overwhelming evidence that natural selection is quite able to keep genomes from deteriorating. It is even capable of enabling previously-deteriorated genomes to improve. There are many exceptions to rules in biology, so there are a few examples in the literature of fitness declining even when selection is operating in a reasonably large population. For instance, we noted earlier a case where Drosophila chromosomes deteriorated in a chilled control population [60]. We would actually expect to observe at least some cases of mutational meltdown in populations, since the fossil record indicates that over 99.9% of all species that ever existed have gone extinct. These cases do not invalid the general rule that the fitness of large populations is maintained just fine by natural selection.
In Sanford’s chapter “Is The Downward Curve Real?” I expected that he would search out a few of these exceptional studies and wave them around as though they were the norm. Instead, all he does in ten pages is present inappropriate analogies and note that the life spans of Biblical characters following Noah decline in a roughly exponential fashion. The Biblical issue is addressed below.
For all Sanford’s hand-wringing over the inescapable declines in genomes everywhere, his lack of concrete examples shows that the scientific facts are not on his side. Microbes have existed for untold millions of generations, and even small mammals like mice and rabbits which reproduce one or more times a year have existed with humans for thousands of generations in historic times, and many more thousands of generations in prehistoric times. If genomes of these rodents were declining by say 0.1% per year, then in 3000 years since 1000 BC, they should be down to 5% of their original fitness (0.999 raised to 3000 power = 0.05) So where is the evidence of super-rabbits in 1000 BC or even 1000 AD ?
Laboratory experiments with Drosophila fruitflies have been going on for a hundred years. At about two weeks per generation, that represents some 2600 generations. To my knowledge, there has not been a systemic decline in viability of the laboratory populations in this timeframe.
The only evidence of genomic deterioration that Sanford cites (in Appendix 1, pg. 175) is a study by Carlsen et al. [64], claiming this shows that “Human fertility and human sperm counts are both now dramatically declining.” This study by Carlsen was actually a “meta-study,” surveying 61 publications between 1938 and 1991. The difficulties in comparing results among different research groups across 5 decades are formidable. Other meta-studies conclude that if there has been a decline in semen quality, it is non-linear and is best explained by environmental factors, not underlying genetics [65]. The most comprehensive and self-consistent direct study on this subject was published by Andolz, et al. [66]. They report results for more than 20,000 men obtained by the same team in the same clinic in the same location (Barcelona area) for 36 years, finding no significant deterioration in sperm quality.
In general, indicators of human physical well-being have risen over the past several centuries, and have likely been fairly steady over at least the past several thousand years. Average human life spans at birth are estimated as 20-30 years for classical Greece and Rome and for medieval Britain, compared to 30-40 years in the early twentieth century and about 65 years now. This measure includes childhood death. [67] Environmental factors such as increases in nutrition and medicine over the last two hundred years likely explain the increase in the past three centuries, but the relatively steady values before 1700-1800 A.D. indicate the human race has not been deteriorating.
Although average life spans have increased, maximum life spans have remained about 105−122 calendar years throughout recorded history [68]. In Old Testament times, 70-80 years was considered a ripe old age (Psalm 90:100). This hasn’t changed a lot since then. All this contradicts Sanford’s assertion that the human genome is deteriorating.
James Crow’s Speculation on Human Genetic Deterioration
Sanford repeatedly refers (pp. 45, 65, 143, 171) to a 1997 article by James Crow [69], in which Crow speculates that human genetic fitness may be deteriorating by 1-2% per year. This sounds dire, but Crow makes it clear that the only reason he makes this suggestion is that modern hygiene and small family sizes have largely shut down natural selection among humans, such that much of the world is now running a gigantic mutation accumulation experiment. In a 2010 article [98], Michael Lynch derived a similar estimate (1% to 5%) of the expected fitness decline per generation for humans in industrial societies where improved nutrition and medical care allow the vast majority of individuals to survive to reproductive age. All would agree that under those circumstances, deleterious mutations will accumulate and fitness will decline at some rate; Crow picked 1-2% simply by analogy with Mukai’s fruitfly studies. Crow makes it clear that this fitness decline is merely a speculation on his part, not based on evidence of actual fitness decline, and that this proposed genetic deterioration only pertains to the last few centuries with greatly reduced rates of infant and childhood mortality, not to mankind’s history in general.
In his first reference to Crow (p. 45), Sanford acknowledges that Crow bases his concern on recent relaxed natural selection. However, that crucial factor is omitted in his subsequent references to Crow, which gives the impression that Crow agrees with Sanford that mutation accumulation is a general problem for the human race. In pp. 171-172, Sanford selects a whole page’s worth of quotes from Crow, focusing on the most alarming sentences (“It seems clear that for the past few centuries harmful mutations have been accumulating. …The decrease in viability from mutation accumulation is some 1 or 2% per generation…I do regard mutation accumulation as a problem. It is something like the population bomb, but it has a much longer fuse…”). Sanford’s commentary on this quote-mine is that Crow “goes on to acknowledge that humanity must now be genetically inferior to our stone-age ancestors – an amazing confession about the reality of genomic degeneration.” This implies that Crow’s work in some way supports Sanford’s contention that the human genome is inevitablydeclining, with or without natural selection in operation. That is grossly misleading.