No. Entropy is a state function. Random, non-random, deterministic, chaotic, quantum, spooky, etc., all processes lead to the same entropy for the same state. This is what Sewell seems to be missing in his appendix.
I stated the second law of thermodynamics and you said "No"?
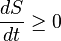
Of course entropy is a state function. It is a measure of the thermodynamic state at a particular point in time. If time x occurs before time y, the state at time x will always be have less or equal entropy to the state of entropy at time y, for a closed system.
"all processes lead to the same entropy for the same state"
The state changes with time. That is like saying the entropy at time x is the same as the entropy time x. Maybe all of your systems are assumed to already be at equlibrium, but even then the statement is a strech. Maybe you have invented a way to stop time (travel the speed of light), but I doubt it.