Posted on 05/23/2007 11:46:25 AM PDT by Red Badger
Researchers at Virginia Tech, Oak Ridge National Laboratory (ORNL), and the University of Georgia have developed a novel method using multiple enzymes as a catalyst for the direct, low-cost production of hydrogen from biomass.
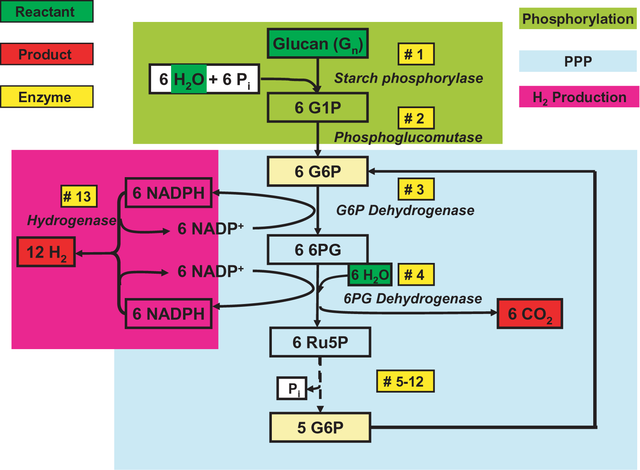
Applying the principles of synthetic biology, the researchers use a combination of 13 enzymes to form an unnatural enzymatic pathway to completely convert polysaccharides—e.g., starch and cellulose—and water into hydrogen at a yield higher than the theoretical yield of biological hydrogen fermentations. Their work is described in the 23 May issue of PLoS ONE, the online, open-access journal from the Public Library of Science.

he synthetic metabolic pathway for conversion of polysaccharides and water to hydrogen and carbon dioxide.

Hydrogen production from either 2 mM G-6-P or 2 mM starch (glucose equivalent) using the new method.
Starch is a high energy-density carrier, with 14.8 H2-based mass%. (The DOE long-term target for hydrogen storage is 12 mass%.) The enzymes, when added to the biomass solution, use the energy in the polysaccharides to break the water up into carbon dioxide and hydrogen.
A membrane bleeds off the carbon dioxide and the hydrogen is used by a fuel cell to create electricity. The water byproduct is recycled for the starch-water reactor. Laboratory tests confirm that it all takes place at low temperature—30° C—and atmospheric pressure. The researchers estimated the cost of hydrogen production using their method of approximately $2/kg.
The stoichiometric reaction is:
C6H10O5 (l) + 7 H2O (l) → 12 H2 (g)+6 CO2 (g)
The overall process is spontaneous and unidirectional because of a negative Gibbs free energy and separation of the gaseous products with the aqueous reactants.
The vision is for the ingredients to be mixed in the fuel tank of a car. A car with an approximately 12-gallon tank could hold 27 kg of starch, which is the equivalent of 4 kg of hydrogen. One kg of starch will produce the same energy output as 1.12 kg (0.38 gallons) of gasoline.
The research was based on earlier work by Y.H. Percival Zhang, assistant professor of biological systems engineering at Virginia Tech pertaining to cellulosic ethanol production (earlier post) and the ORNL and University of Georgia researchers' work with enzymatic hydrogen production.
One of the team, Michael W.W. Adams of the University of Georgia UGA, is co-author of the first enzymatic hydrogen paper in Nature Biotechnology in 1996. The researchers were certain they could combine the processes.
In nature, most hydrogen is produced from anaerobic fermentation. But hydrogen, along with acetic acid, is a co-product and the hydrogen yield is pretty low—only four molecules per molecule of glucose. In our process, hydrogen is the main product and hydrogen yields are three-times higher, and the likely production costs are low—about $1 per pound of hydrogen.
What is more important, the energy conversion efficiency from the sugar-hydrogen-fuel cell system is extremely high—greater than three times higher than a sugar-ethanol-internal combustion engine. It means that if about 30 percent of transportation fuel can be replaced by ethanol from biomass as the DOE proposed, the same amount of biomass will be sufficient to provide 100 percent of vehicle transportation fuel through this technology. —Y.H. Percival Zhang
The next step for the team is to increase reaction rates and reduce enzyme costs.
Resources:
*
“High-Yield Hydrogen Production from Starch and Water by a Synthetic Enzymatic Pathway”; Y.H. Percival Zhang, Barbara R. Evans, Jonathan R. Mielenz, Robert C. Hopkins, Michael W.W. Adams; PLoS ONE 2(5): e456. doi:10.1371/journal.pone.0000456
And some places even pay you to take their waste products. :)
I’d appreciate some help from someone better at chemistry than I am. (Which ain’t hard.)
Are they trying to say that the starch is completely converted to CO2 and hydrogen? That there is no solid or liquid residue left at all?
You replied to the wrong person.
And cornstalks are biomass...
Couldn’t you also use a crop chosen for nitrogen fixation for biomass production, rotating it with other crops to help improve soil conditions?
We used to plant a winter crop of legumes to ‘fix’ the nitrogen in the soil and then would plow it under for the humus. We could have done a summer crop of cotton and then a winter crop of beans but the beans weren’t worth the price to harvest. Fertilizer was expensive when we were doing this so the free nitrogen and humus, not to mention the soil cover, offset the cost of planting beans.
Water byproduct, but otherwise, it looks like all gas!.........
Chick Peas make a great hummus............
Sorry!

MMMMMMMMMMM!....HUMMUS!...........
http://en.wikipedia.org/wiki/Hummus
Ha! I knew I should not have skipped lunch! That extra ‘m’ makes all the difference. :)
Thanks. I was envisioning the buildup of some kind of sludge in the gas tank, and trying to figure out how you’d go about cleaning it out. :)
I make my own hummus. Storebought just don’t taste right........
Sewage would also be biomass.
And not particularly tasty.
I make my own humus....and the plants seem to find it tasty. :)
I was thinking the same thing. This would basically be a sh!t eating, fart producing hydro machine suitable for putting at the city dump and sewage treatment facilities.
Controlling the reaction seems to be an issue. If you fill ‘er up with starch, you immediately start producing hydrogen. There needs to be a way to draw it off and store it (compressed?) even when the vehicle isn’t powered up.
What happens if you don’t get pure enough starch or enzymes, or you get the ratios mixed up? You slow down the reaction and could run out of fuel, even though you’ve got some in the tank. Lots of issues here, I think. But a nice start!
Non-comestible biomass can be grown on terrain unsuitable for food crops. A great deal of lumber is harvested from such terrain.
Forgive my naivete, but it looks like the enzyme reaction, freeing the Hydrogen molecules, takes place in the Phase #3 portion of the process. This may provide a natural petcock point for shutting down the reaction by denying fuel.
Remembering that this is conceived as a fuel cell application, not combustion, the fuel cell could still receive excess Hydrogen ions from the ongoing reaction.
What's left, if there is still excess could either be contained at low pressure, or drawn off onto the grid or a storage battery.
Perhaps I only prove that ignoraqnce is bliss, eh?
The Sunflower’s petals hold glass coils of photosynthetic algae and the resulting “sugars” are siphoned off. It also uses very little added water, which might become the next limiting quantity.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.