Posted on 01/02/2011 9:04:23 PM PST by neverdem
Chinese researchers have developed a magnetic solid acid catalyst that raises the prospect of efficiently converting biomass cellulose into useful chemicals, such as sugars for biofuel production.
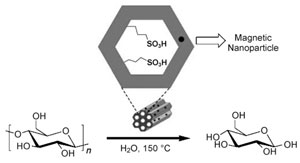
According to the researchers, the catalyst is better than conventional equivalents because it shows good hydrothermal stability and can be recycled - magnetic nanoparticles pull the acid away from the substrate when a magnetic field is applied.
Using biomass as a source of renewable fuel has attracted interest in recent years in response to global climate change and the search for alternatives to fossil fuels. The main component of biomass is cellulose - a polymer comprising many glucose units linked by beta-1,4-glycosidic bonds, with each chain then interconnected by hydrogen bonds. This structure makes cellulose a tough compound to break down. Enzymes or acid catalysts are needed to do the job. But then separating the catalyst from the reaction residue can be energy intensive and costly.
Now, Yao Fu, at the University of Science and Technology of China in Hefei, and colleagues have come up with an answer. Inspired by previous work that showed sulfonic acid functionalised mesoporous silica materials work well as acid catalysts,1 the team designed and synthesised their new sulfonic acid catalyst in the presence of magnetic Fe3O4 nanoparticles, triblock copolymers and hydrogen peroxide.2

|
The magnetic solid acid catalyst improves the hydrolysis of cellulose to form glucose
© ChemSusChem
|
'By using our new catalyst, we saved an energy-consuming process to separate the solid catalyst and concentrate the glucose solution,' says Fu. The team tested the catalyst by studying the hydrolysis under different conditions of various carbohydrates, including: cellobiose, starch, cellulose and lignocellulose from corn cobs. They found their sulfonic acid effectively hydrolysed 1,4-glycosidic bonds, producing glucose yields of up to 96 per cent from cellobiose, a disaccharide comprising two glucose molecules. However, only 50 per cent yields were obtained from amorphous cellulose. Importantly, the acid could be used repeatedly without deactivation.
'Developing a heterogeneous catalyst for cellulose hydrolysis has been a goal for many scientists,' says Joseph Binder, who researches biofuel chemistry at the University of California Berkeley, US. He thinks the enhanced separation and stability properties of the new catalyst are an encouraging contribution towards this goal. 'Still, the challenge of achieving high yields of glucose through the action of a solid catalyst on the insoluble cellulose substrate remains to be solved,' he adds.
Fu acknowledges that further work needs to be done to realise the industrial potential. 'The optimisation of hydrolysis conditions and low cost catalyst synthesis need to be overcome,' he says. But after modifications have been made to the process and reactor, Fu is optimistic that the process of hydrolysis of cellulose using a magnetic acid catalyst can be scaled up to industrial level.
1. D Margolese et al, Chem. Mater., 2000, 12, 2448
2. D Lai et al, ChemSusChem, DOI: 10.1002/cssc.201000300
Wonder what this does to nanotech?
/johnny
bookmark
I think it does more catalyst recovery and biofuel. Cellulose is almost everywhere for the taking except high altitudes and latitudes.
But the binding thing.... that's important. That's what I'm looking at.
/johnny
What are you binding?
You expect something like iridium?
I'm a cook/network engineer or network engineer/cook, depending on the economy.
I know carbon.
/johnny
As long as we don’t have to subsidize it, or be forced to put ethanol in our cars, I’m all for it.
/johnny
Last time somebody said “Yao Fu” to me I almost hit ‘em.
I put one of those magnetic thingys on my carbuerator and still haven’t got 100 MPG.
That pyramid under the bed doesn’t work either.
s/ off
Actually, this "is" nanotech. Binding selectively reactive entities (antibodies and similar) to magnetic beads is one of the BIG ways of increasing selectivity and sensitivity of biochemical analysis. This is the first use I've heard of it being used in PRODUCTION of chemicals, especially bulk chemicals, but the technology itself has been around a while.
A nice, innovative piece of work.
Risk for alcoholism linked to risk for obesity
Bacteria's Viral DNA Offers a Sneak Peek into Primitive Immune Systems
A Diet Manifesto: Drop the Apple and Walk Away That title reminds me of the NRA's Eddie Eagle program.
FReepmail me if you want on or off my health and science ping list. Happy New Year!
The most common organic molecule on the planet, by far.
I’ve read that as much as 30% of the biomass on earth is cellulose.
...a magnetic solid acid... shows good hydrothermal stability and can be recycled - magnetic nanoparticles pull the acid away from the substrate when a magnetic field is applied.Thanks neverdem.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.