Posted on 01/18/2012 12:02:09 AM PST by neverdem
Highly sensitive explosives could become safer and greener by exploiting newly characterised ionic polymer structures, say chemists in the US. Such materials could replace explosives based on toxic heavy metals like lead and mercury salts.
Sensitive materials are routinely used as primary explosives in detonators to set off larger amounts of less sensitive high explosives in mining or military applications. The challenge is to make them stable enough to be handled safely in the field, but also sensitive enough to detonate reliably, packing as much energetic punch as possible. 'It's a very fine balance,' says Louisa Hope-Weeks of Texas Tech University (TTU) in Lubbock, who led the research.
'We wanted to make an optically activated material,' explains Hope-Weeks, 'so that if, for example, a bomb squad want to blow up a car they suspect has a terrorist device inside, they would have something they could remotely place under the car and then activate it with a laser, rather than someone having to go in there and wire it up.'
While none of the materials the group has so far made has reached this goal, by making and solving the x-ray crystal structures of three materials based on metal hydrazine polymers, the team can relate the materials' properties to their structures.
However, this kind of research obviously comes with significant hazards. These compounds are the same ones that caused a serious explosion in Hope-Weeks's lab in 2010, seriously injuring Preston Brown, one of the contributors on this paper. 'There was a lot learned from that accident, both from the students' point of view and my own,' comments Hope-Weeks. 'I've changed the way I manage my whole lab, and TTU has responded across the board.'
All this made for some nerve-racking moments in the crystallography lab. 'In the case of nickel hydrazine perchlorate, which we knew was very sensitive, we did need to cut a crystal to get one the right size,' Hope-Weeks says. 'I did that myself.' However, she points out that no particularly special crystallographic equipment was needed, since the size of crystals used - had they exploded - would not have caused significant damage to the diffractometer.
Thomas Klapötke, an energetic materials expert from the University of Munich in Germany, is impressed by the team's efforts at crystallising and characterising the materials. 'This is not easy, as these kinds of polymers often tend to crash out of solution as powders,' he says.

|
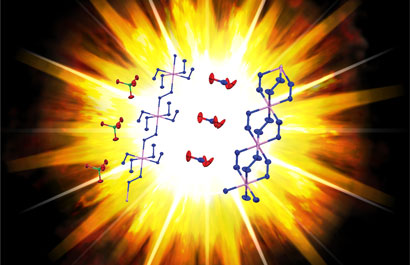
Structural differences between nickel hydrazine perchlorate (left) and nickel hydrazine nitrate (right) can explain their sensitivity and energetic properties
© Adapted from J. Am. Chem. Soc.
|
The knowledge Hope-Weeks has gained should help her understand, tune and refine the stability and energetic properties of related compounds. For example, in nickel hydrazine nitrate - which is already used as a primary explosive, but whose crystal structure was unknown - each metal atom is linked to the next by three bridging hydrazine ligands. However, in nickel hydrazine perchlorate, only one hydrazine bridges between metal centres, with four more hydrazines coordinated to each nickel. This means the whole polymer is less strongly held together, making it significantly more sensitive. And when it does explode it can release more energy, because of the potential to produce more nitrogen gas per metal atom and form more thermodynamically stable products like nickel chloride rather than nickel metal. Changing the metal to cobalt produced a material with the same structure, but that was significantly less sensitive
One of the advantages of this class of materials is their tunability, explains Hope-Weeks. 'We can try different metals, different anions and other ligands,' she says, 'which might allow us to tweak the properties just enough.'
Klapötke agrees that there are many options to explore. 'These ionic polymers are very interesting, especially because they are not hygroscopic and non-toxic,' he points out. 'This gives them a long shelf-life because they don't react with water, which might alter their sensitivity and make them unreliable.'
O S Bushuyev et al, J. Am. Chem. Soc., 2012, DOI: 10.1021/ja209640k
10 Surprising Health Benefits of Beer
Red-Wine Researcher Charged With 'Photoshop' Fraud (Accused Dr. blames "racial hatred")
Diet, nutrient levels linked to cognitive ability, brain shrinkage
FReepmail me if you want on or off my health and science ping list.
1. green bullets
2. green fuel
3. gay sensitivity training for US Marines in the field
4. green explosives
Ummm......I see a pattern.

The sulfate anion, that's the yellow sulphur atom bound to four red oxygen atoms, has a charge of minus two. The perchlorate anion has a charge of minus one. So my guess is that there are twice as many perchlorate anions in nickel hydrazine perchlorate that use an oxygen to oxygen bond, like you find in hydrogen peroxide, bonding adjacent perchlorate anions, IMHO, so that the net charge of the molecule is zero. (The pale green Ni atoms are nickel. The dark blue N atoms are nitrogen. The unmarked dark atoms bound to nitrogen are hydrogen.)
What Is A Coordination Compound?
Perchlorates can be very strong oxidizing agents, e.g. The mixture of a perchlorate and any organic material may result in explosion that may be touched off by friction...
I have.
From a shooting perspective, they went "bang" every time, hit the target, and showed the same muzzle velocity for bullet weight as similar lead styphnate primed ammunition.
From a reloader's perspective ... looks like I don't have to tumble the brass. It comes out of the weapon already clean.
I was really worried about that. There was a news story online about how the Russians have built an even more powerful version of the US’ MOAB, and tried to sell it as green technology by pointing out that it doesn’t generate nuclear fallout.
It also isn’t very powerful compared with a nuke, of course. And it’s not as if they’ve scrapped their nuclear arsenal.
Thanks neverdem.
I have more family than I realized!
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.