Posted on 03/25/2011 4:24:17 PM PDT by neverdem
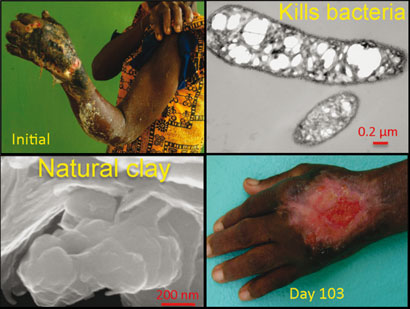
US researchers have made a step towards understanding why some natural clays are antibacterial, boosting the chances that they could one day be used as alternatives to antibiotic drugs. According to the researchers, the clays supply iron that kills bacteria by generating radicals that attack cell components.
People have used clays throughout history for healing. More recently, patients in Ivory Coast suffering from a flesh-eating disease known as Buruli ulcer were treated with French green clays. The clays appeared to ease the swelling of the lesions like an antibiotic, but no-one knew how they did it.
Lynda Williams and colleagues at Arizona State University in Tempe came to this mystery in 2002. They found that in fact just one of the French clays was antibacterial, and that even then the clay's antibacterial properties were not consistent from batch to batch. 'Because of this we have been studying what makes a natural clay antibacterial,' says Williams.

|
Buruli ulcer, a flesh-eating bacterial infection, can be treated with certain iron-rich clays. The iron infiltrates the cells and generates lethal radicals
© Environ. Sci. Technol.
|
Williams and colleagues have now made a crucial step in that direction. They have analysed clay supplied by an open-pit mine in the volcanic Cascade mountains in Oregon, US, which is the most effective antibacterial clay the researchers have come across. Using x-ray spectroscopy and inductively coupled plasma mass spectrometry, the researchers studied the clay's mineralogy, its composition, and the chemistry of both the clay and nearby E. coli bacteria in water.
Compared with control experiments, in which non-antibacterial clay was dispersed in water with E. coli, the concentration of iron inside the bacteria near to the antibacterial clay was eight times higher. Williams's group therefore says that iron is the 'primary reactant' in the antibacterial process. They believe that Fe<sup>2+</sup> ions overwhelm the bacteria's outer membranes, oxidising inside the cells to produce lethal hydroxyl (OH) radicals.
'I think the results should contribute to the understanding of antimicrobial behaviour of nanoparticles,' says Yulong Ding, a bioengineer at the University of Leeds who studies naturally antibacterial nanoparticles. However, Ding adds that scientists should probably interpret the finding 'as one of several possible mechanisms.'
L B Williams et al, Environ. Sci. Technol., 2011, DOI: 10.1021/es1040688
micro ping
Interesting.
Smectic clay is indeed antimicrobial both taken internally and smeared topically. These clays look (on microscopic scale) like a stack of pancakes on an stick with space maintained between the pancakes. On a molecular level it it sharp and has been shown capable of lysing bacterial cell membranes. Thats how they thought it worked.
This report describes how there is a lot of iron on the center of these "pancakes" and the iron generates nasty radicals. Ok sounds good to me.
you have no idea how many things are found to have totally different mechanisms of action that is what is commonly accepted. Science is loads of fun.
This is a nice looking post, btw!
Eat Clay!
Silver is effective against well over 500 different types of Bacteria!!
The iron in hemoglobin isn’t free to create Fe+ ions which appear to be the reason for the clays’ antiseptic qualities.
Fe isn’t hard to come by in the environment, but the physical properties of the clay help keep it on the skin and with sufficient water to enable mobility of the iron ions. A lotion of something like iron oxide (e.g. calamine) also has these ions, but it would quickly dry and flake off.
Granted, there's a downside to storing iron...

I see several twists and turns to this.
First of all, one of the most effective therapeutic clays is Bentonite.
http://en.wikipedia.org/wiki/Bentonite
Bentonite is an absorbent aluminium phyllosilicate. There are different types of bentonites, and their names depend on the dominant elements, such as potassium (K), sodium (Na), calcium (Ca), and aluminum (Al).
But, no iron.
Second, several ionic metals are known to inhibit bacterial reproduction, but do not harm the bacteria itself.
Thanks for your links.
. for later
Some important notes about ionic elements and their use against bacteria and viruses.
To start with, ionic elements need to be classed by their interactions with both bacteria and humans.
Some elements, such as oxygen, chlorine, calcium, potassium, sodium, etc., are generally lethal to bacteria, and may or may not be dangerous or harmful to humans, given the context.
Others are not generally lethal to bacteria and viruses, but can inhibit their reproduction, such as silver and zinc. The colloidal, or small molecules held in suspension in a liquid, version of these were ignored for many years, because they were not immediately lethal, though anecdotally they seemed to be effective in context.
But another twist was that neither silver nor zinc are typically readily uptaken into the mucous membranes, where they would be of most use. This has been changed in recent years by the discovery of a form of zinc, zinc gluconate glycine, is readily uptaken and has been proven to reduce both duration and severity of upper respiratory colds.
Third is a group of elements that are hostile to both bacteria and humans. A good example is the semi-metal arsenic, which has been shown to inhibit the body’s recognition of invading pathogens, so they are established before the immune system starts fighting them. Then the immune system overreacts, possibly harming the victim.
 . with lead. :))
. with lead. :))
Kaopectate used to be made with a natural clay ingredient.
Till the meddling retarded FDA forced them to change it to the same stuff as Pepto Bismuth.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.