|
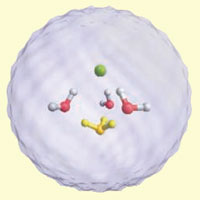
Calculated structure for the separated ion pair (Cl- green, H2O red and white, H3O+ yellow)
© Science
|
Posted on 06/24/2009 9:10:06 AM PDT by neverdem
Scientists in Germany have observed a single molecule of HCl dissociating into its component ions in water - and have discovered that just four water molecules are needed for complete dissociation of the acid. The team say that their findings, made under ultracold conditions, should help scientists understand nanoscale chemical transformations at very low temperatures, including those occurring in stratospheric clouds and interstellar media.
Previous studies into the dissociation mechanism of the strong acid HCl at ultracold temperatures had left a puzzle. Normally, such reactions require thermal energy, but at ultracold temperatures this thermal energy is not available. Now, Martina Havenith and colleagues at the Ruhr-University Bochum, Germany, have solved the puzzle.
They found that just four water molecules are required to obtain charge separation of HCl into a positive hydronium ion and a negative Cl- ion. Hydronium ions are an important ingredient in many chemical reactions but until now it was not clear how many water molecules are required to complete this charge separation. What's more, a new reaction mechanism was found that explains dissociation even at ultracold temperatures which the team named 'aggregation induced dissociation'.

|
Calculated structure for the separated ion pair (Cl- green, H2O red and white, H3O+ yellow)
© Science
|
'Our results provide a route for the understanding of chemistry at cryogenic and ultracold temperatures,' says Martina Havenith, who led the study. 'The results are of interest for a wide community including chemistry, astronomy, atmospheric science and physics, where the field of ultracold reactions is rapidly gaining interest,' she adds.
The team made their discovery by embedding HCl and individual water molecules in an ultracold trap composed of nanodroplets of superfluid helium - which have a temperature of less than -272.8°C (0.37K). Having trapped the molecules within the helium droplets, the team used infrared laser spectroscopy to look for the spectral peak corresponding to the H3O+ ion - finding the signal in clusters of just four water molecules.
'Understanding how acids undergo their prototypical reaction from neutral (HCl) to ionized species in water is central to much of chemistry because acid-base chemistry is so pervasive to what we do,' says Timothy Zwier, a physical chemist at Purdue University, West Lafayette, US.
'Others have searched for such zwitterion [electrically neutral compound that carries positive and negative charges on different atoms] formation in molecular clusters unsuccessfully, but theoreticians had predicted the possibility that only four water molecules might suffice for the reaction to proceed spontaneously,' adds Zwier. 'The work from the Havenith group provides evidence that indeed this is the quintessential smallest droplet of water in which HCl can undergo its acid-base reaction,' he says.
A Gutberlet et al, Science, 2009, 324, 1545 (DOI: 10.1126/science.1171753)
Far out!
And the ‘K’ constant for HCL is..................................??????
If you mean dissociation equilibrium constant, then you have as much info as I do. Otherwise, K refers to degrees of temperature using the Kelvin scale.
heh ;)
I heard they’re putting them on the back of postage stamps for unsuspecting people to lick.
I also wonder how they performed the calculations done in conjunction with their spectroscopy experiments. Something like MD-DFT (if they used a black-box package, this is almost guaranteed to be the flavor of calculation performed) holds well for many systems at ambient conditions, but you generally need to use something more accurate at very low temperatures, especially if the key reaction involves a low mass constituent, or else you may miss the lowest energy reaction coordinate.
Warp Drive Engine Could Suck Earth Into Black Hole
The Weirdest Object in the Solar System?
FReepmail me if you want on or off my health and science ping list. Anyone can post any unposted link as they see fit.
their findings, made under ultracold conditions, should help scientists understand nanoscale chemical transformations at very low temperatures, including those occurring in stratospheric clouds and interstellar media.Thanks neverdem. Okay, this is list-related, but we probably have to squint our eyes really hard to see it. ;')
 |
||
| · join · view topics · view or post blog · bookmark · post new topic · | ||
| Google news searches: exoplanet · exosolar · extrasolar · | ||
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.