Posted on 05/30/2023 11:54:00 AM PDT by Red Badger

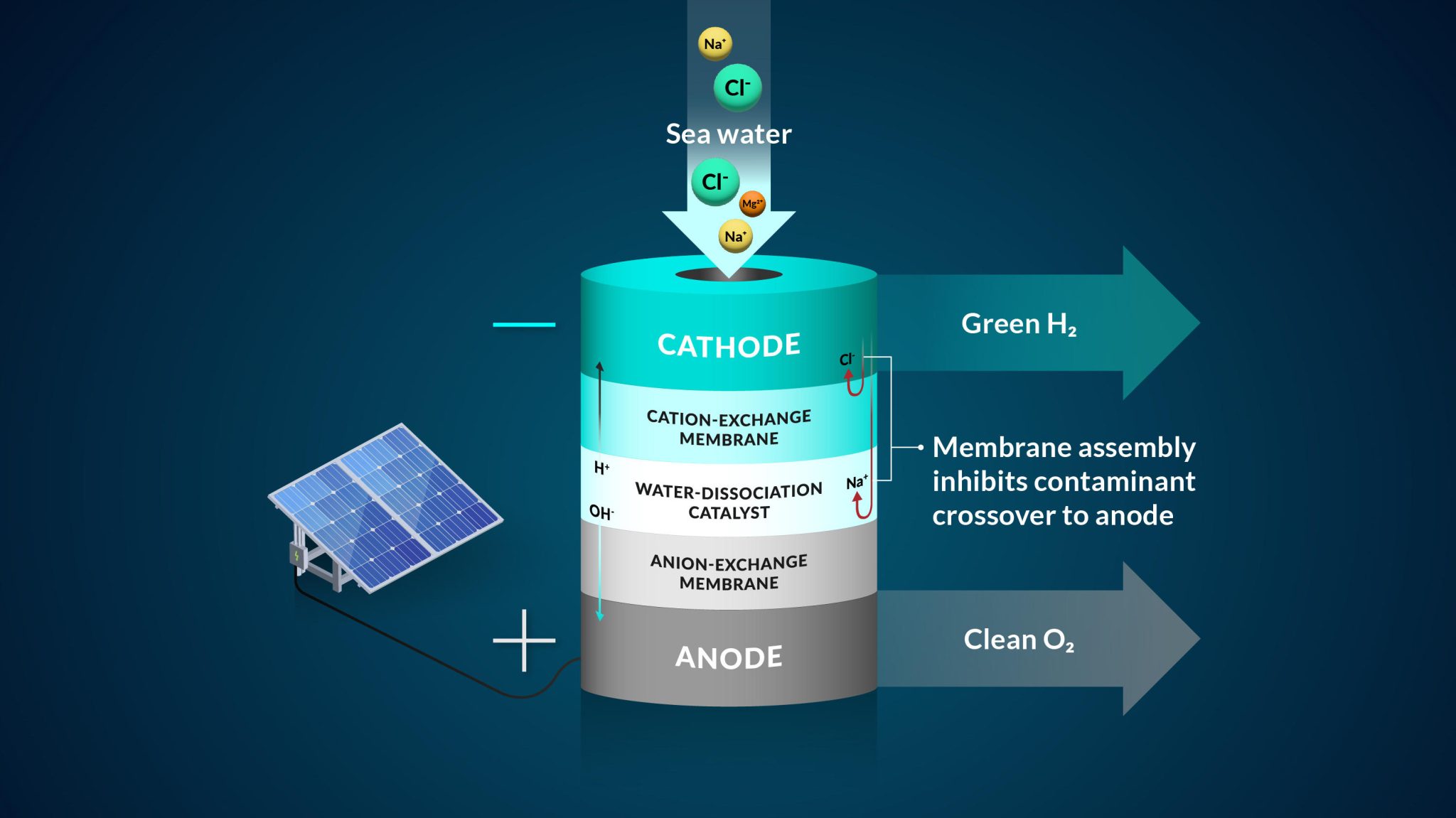
A representation of the team’s bipolar membrane system that converts seawater into hydrogen gas. Credit: Nina Fujikawa/SLAC National Accelerator Laboratory
The cocktail of elements in seawater, including hydrogen, oxygen, sodium, and others, is essential for life on Earth. However, this intricate chemical makeup poses a challenge when attempting to separate hydrogen gas for sustainable energy applications.
Recently, a team of scientists from the Department of Energy’s SLAC National Accelerator Laboratory, Stanford University, University of Oregon, and Manchester Metropolitan University has discovered a method to extract hydrogen from the ocean. They accomplish this by funneling seawater through a double-membrane system and electricity.
Their innovative design proved successful in generating hydrogen gas without producing large amounts of harmful byproducts. The results of their study, recently published in the journal Joule, could help advance efforts to produce low-carbon fuels.
“Many water-to-hydrogen systems today try to use a monolayer or single-layer membrane. Our study brought two layers together,” said Adam Nielander, an associate staff scientist with the SUNCAT Center for Interface Science and Catalysis, a SLAC-Stanford joint institute. “These membrane architectures allowed us to control the way ions in seawater moved in our experiment.”
Hydrogen gas is a low-carbon fuel currently used in many ways, such as to run fuel-cell electric vehicles and as a long-duration energy storage option – one that is suited to store energy for weeks, months, or longer – for electric grids.
Many attempts to make hydrogen gas start with fresh or desalinated water, but those methods can be expensive and energy intensive. Treated water is easier to work with because it has less stuff – chemical elements or molecules – floating around. However, purifying water is expensive, requires energy, and adds complexity to devices, the researchers said. Another option, natural freshwater, also contains a number of impurities that are problematic for modern technology, in addition to being a more limited resource on the planet, they said.
To work with seawater, the team implemented a bipolar, or two-layer, membrane system and tested it using electrolysis, a method that uses electricity to drive ions, or charged elements, to run a desired reaction. They started their design by controlling the most harmful element to the seawater system – chloride, said Joseph Perryman, a SLAC and Stanford postdoctoral researcher.
“There are many reactive species in seawater that can interfere with the water-to-hydrogen reaction, and the sodium chloride that makes seawater salty is one of the main culprits,” Perryman said. “In particular, chloride that gets to the anode and oxidizes will reduce the lifetime of an electrolysis system and can actually become unsafe due to the toxic nature of the oxidation products that include molecular chlorine and bleach.”
The bipolar membrane in the experiment allows access to the conditions needed to make hydrogen gas and mitigates chloride from getting to the reaction center.
“We are essentially doubling up on ways to stop this chloride reaction,” Perryman said.
A home for hydrogen The ideal membrane system would perform three primary functions: separate hydrogen and oxygen gases from seawater; help move only the useful hydrogen and hydroxide ions while restricting other seawater ions; and help prevent undesired reactions. Capturing all three of these together is hard, and the team’s research is targeted toward exploring systems that can efficiently combine all three of these needs.
Specifically in their experiment, protons, which were the positive hydrogen ions, pass through one of the membrane layers to a place where they can be collected and turned into hydrogen gas by interacting with a negatively charged electrode. The second membrane in the system allows only negative ions, such as chloride, to travel through.
As an additional backstop, one membrane layer contains negatively charged groups that are fixed to the membrane, which makes it harder for other negatively charged ions, like chloride, to move to places where they shouldn’t be, said Daniela Marin, a Stanford graduate student in chemical engineering and co-author. The negatively-charged membrane proved to be highly efficient in blocking almost all of the chloride ions in the team’s experiments, and their system operated without generating toxic byproducts like bleach and chlorine.
Along with designing a seawater-to-hydrogen membrane system, the study also provides a better general understanding of how seawater ions move through membranes, the researchers said. This knowledge can help scientists design stronger membranes for other applications as well, such as producing oxygen gas.
“There is also some interest in using electrolysis to produce oxygen,” Marin said. “Understanding ion flow and conversion in our bipolar membrane system is critical for this effort, too. Along with producing hydrogen in our experiment, we also showed how to use the bipolar membrane to generate oxygen gas.”
Next, the team plans to improve their electrodes and membranes by building them with materials that are more abundant and easily mined. This design improvement could make the electrolysis system easier to scale to a size needed to generate hydrogen for energy-intensive activities, like the transportation sector, the team said.
The researchers also hope to take their electrolysis cells to SLAC’s Stanford Synchrotron Radiation Lightsource (SSRL), where they can study the atomic structure of catalysts and membranes using the facility’s intense X-rays.
“The future is bright for green hydrogen technologies,” said Thomas Jaramillo, professor at SLAC and Stanford and director of SUNCAT. “The fundamental insights we are gaining are key to informing future innovations for improved performance, durability, and scalability of this technology.”
Reference: “Hydrogen production with seawater-resilient bipolar membrane electrolyzers” by Daniela H. Marin, Joseph T. Perryman, McKenzie A. Hubert, Grace A. Lindquist, Lihaokun Chen, Ashton M. Aleman, Gaurav A. Kamat, Valerie A. Niemann, Michaela Burke Stevens, Yagya N. Regmi, Shannon W. Boettcher, Adam C. Nielander and Thomas F. Jaramillo, 11 April 2023, Joule. DOI: 10.1016/j.joule.2023.03.005
This project is supported by the U.S. Office of Naval Research; the Stanford Doerr School of Sustainability Accelerator; the DOE’s Office of Basic Energy Sciences, Chemical Sciences, Geosciences, and Biosciences Division through the SUNCAT Center for Interface Science and Catalysis, a SLAC-Stanford joint institute; and the DOE’s Energy Efficiency and Renewable Energy Fuel Cell Technologies Office.
They don’t mention whether it takes more energy than it produces.
And I’m betting IT DOES.
I once spent a week in a EPICS class with mostly SLAC Post docs. They are smart kids, but not very practical.
I think I got an A- in the class.
They are dreamers looking for the unknown. I’m an engineer that dealt with reality.
Reality is this, “fossil” fuels at this point of our technology make the most sense for the well being of our present society, Pie in the sky Non-Carbon BS is just a method of controlling idiots that vote for Democrats.
Sorry but there is nothing wrong with coal, oil, or natural gas.
There is something very wrong with people that want to destroy our economy just to enslave us to some un-obtainable ideal of a pollution free world.
Do you defecate? Then you pollute by your very existence.
Please check out and save the Earth.
From the tiny solar panel in the picture...
Ahh.. Yes...
The immutable effects of a government-controlled education system that features science derived using million-man math...
Someone should tell these boffins that Hydrogen is not an energy source...............
The main problem with deep-ocean reverse osmosis systems has been, rapid degradation of the membranes. Anyway, nice find!
It’s never gonna work because the DS/Globalist’s are going to destroy the Electrical Grid.
It's a first law of thermodynamics certainty that it takes more energy than it produces.
The only thing this might be useful for is to store energy from intermittent sources such as wind and solar in the form of hydrogen for future use.
It would be pointless to use fossil fuel electricity for this process, unless the hydrogen itself is the desirable end product for use in hydrogen-fueled vehicles. But even then, it is more efficient to derive hydrogen from natural gas than it is from the electrolysis of sea water.
Exactly. I looked at hydrogen electrolyzers and fuel cells for home energy storage in case the defecation hits the oscillator (as Pop used to put it). And the best I could come up with is a 50% loss of energy round trip. (For every 100kWh of power you use to run the electrolyzer and store hydrogen into a tank, you get only 50kWh out of it from powering a fuel cell.) That's horrible from a grid support perspective.
Then only thing such a system brings to the table is its ability to expand storage cheaply (by bigger or more tanks) relative to more battery storage (much higher efficiency in charging/discharging, but more expensive to add kWh's of storage). For example, a hydrogen tank like in the Toyota Mirai is 32 gallons in size and store at pressure up to 10,000 psi. That gives it a 5kg capacity by weight of H2. The fuel cell in the Mirai converts that to 280kWh, which is more than the total power I pulled from the grid last month to power my house (including charge my EV) because our solar combined with battery storage took care of the rest.
Let's see how an electrolyzer and fuel cell would work, at least for my situation. This is from the perspective of trying to be self-sufficient so that the government's energy policies have less effect on an individual or family. Last month I had 125 hours in which my home battery stack was fully charged and I had nowhere useful for incoming solar power to go (I'm not selling power to the grid). If I had a hydrogen electrolyzer I could at that point configure my inverters to run the electrolzyer and generate hydrogen gas (in a tank I'd store outside down the hill in the ground like in a storm shelter). Then at times my home batteries were drained and my inverters, therefore, pulled whatever power I needed from the grid, my inverters could have instead chosen to first run a power cell to produce power from the stored hydrogen. I already have some experience with a similar system because this is exactly how we usually charge our EV. On days the EV is charged more than enough to handle the next day's chores, we plug it into an intermittent outlet that's powered by our inverters only on the condition that the home batteries are charged more than enough to power the home through the night. Thus, power to charge the EV on those days is charging the EV for future days' worth of driving beyond tomorrow (in case we have little free solar power coming after today). If we don't have excess power today (bad day for solar) the intermittent charger isn't hot and, therefore, doesn't charge the EV. (On those times we're cool with that or we would plug the EV into the constantly powered outlet because we need more charge for the next day's driving even if it means pulling some of that power from the grid.) If I had an electrolyzer to produce hydrogen, I'd power it from the same intermittent panel that the intermittent EV charger outlet is powered.
If I wanted enough hydrogen storage to last the winter without pulling from the grid, I'd just add more tanks without having to also add more expensive equipment (extra electrolyzers and fuel cells). I could fill the tanks in the spring, summer, and fall when I have plenty of excess solar power and hardly need power beyond what my solar and battery storage provide. In the winter I'd consume the hydrogen on nights I don't have enough battery power to heat the home (and charge the EV and in some case entertain guests and consume even more power like during Christmas holidays of if we're all gathered to watch football in January, particularly if Bama makes the playoffs). Though even in winter I have some good solar days that are also low consumption days and, therefore, would have some days I'd run the electrolyzer and build up hydrogen gas. If my fuel cell was as efficient as the Toyota Mirai's fuel cell, I'd need about 7 or 8 of the tanks like the Mirai has (or about 250 gallons if the same psi) and I'd be off-grid without my wife and I decreasing our lifestyle. By comparison, a small propane tank for a home ("small" for the tanks that sit horizontally on the ground) is about 250 gallons.
“their system operated without generating toxic byproducts like bleach and chlorine.”
Sea water is Sodium Chloride dissolved in water. Where does the chorine go when they are done with their process to extract H2 and O2?
I assume it meets back up with a sodium atom................
revolutionary! the geniuses at SLAC have combined an RO membrane with good ol’ electric hydrolysis! ... i bet that combo is so obvious that it’s not even patentable ...
and, GUARANTEED to output less energy than input ...
of course, turning hydrogen into a portable fuel is the usual ball of wax: EXTREMELY reactive material, that is EXTREMELY difficult to contain in leakproof containers, and EXTREMELY difficult to transform into an energy-dense fuel source ...
Here’s an idea!
Let’s store it in lithium tanks!...................
Our roads can always use lots more rock salt. The car makers will be happy.
That's the wrong question to ask. The right question is, "How do you get that grant?"
If I remember my chemistry propane is C3H8 and nat gas is C2H4
Hydro or nuclear power could be nice source for green hydrogen in large quantities.
I believe Plug Power is building a large hydrogen production facility in up-state NY using hydro power to make it green.
The future is brighter for advance nuclear reactors. From the March 4, 2020, edition of Sci Tech Daily, “The U.S. Department of Energy’s Oak Ridge National Laboratory and the Tennessee Valley Authority have signed a memorandum of understanding to evaluate a new generation of flexible, cost-effective advanced nuclear reactors.”
“It still needs energy to pump the water through the membrane and electricity. .
How do you get that electricity?”
In a situation where you do not care what time of day it works; so long as it works some of the day, you could potentially use solar or wind power. Since it is using seawater a tidal turbine is a possibility. Nuclear would be better. The idea is to product transportable energy.
You are exactly correct and if the person who wrote the original article was even minimally competent, that’s what the focus of the article should have been.
If one is worried about CO2 emissions (I’m not), the major problem with windmills and solar panels is their intermittent nature - even if you overbuild by a factor of 5 or 10 relative to carbon fuel sources, on a still night with heaters or air conditioners running, you’re ‘sh*t outta luck’ if you don’t have back-up for those systems. Adequate battery back-up is prohibitively expensive (at least for now), so storing excess wind/solar electricity as chemical energy (that is, H2 and O2 in this case) could be of value.
Except electrolysis has pretty poor efficiency, and storing H2 is problematic. This article explores a modest technical improvement in the electrolysis process (and most technology advances in such small steps) - but as others have noted, it’s no breakthrough and far from any panacea.
Disclaimer: Opinions posted on Free Republic are those of the individual posters and do not necessarily represent the opinion of Free Republic or its management. All materials posted herein are protected by copyright law and the exemption for fair use of copyrighted works.